Stem Cell Therapy for Facial Bone Regeneration
Abstract
Craniofacial abnormalities (CFA) are a varied collection of facial and head development defects. Since a single factor does not cause these defects, many variables contribute, such as genetic disorders, environmentally exposed exposure, and inborn folic acid deficiency, throughout pregnancy. Several distinct kinds of craniofacial surgery include cleft lip and cleft palate repair, Hemifacial microsomia, Vascular malformation, craniosynostosis Hemangioma, and distraction osteogenesis Trigonocephaly. While surgical methods have long been used in this area, the amount of craniomaxillofacial shape and function that may be restored is limited. Regenerative medicine and surgery have given people with various tissue deficiencies the opportunity to reconstruct and regenerate their defects. More recently, stem cell therapy has become an important component of targeted craniofacial bone and cartilage repair. This paper seeks to find the solution to craniofacial disorders using the MSCs therapy for facial jawbone regeneration.
Chapter 1
Statement of the problem
Stem cells showed advantages in treating cardiovascular diseases, incisions and wounds, neurodegenerative diseases, autoimmune diseases, and orthopedic conditions. Despite medical and scientific progress, stem cell problems, such as genetic instability, stem cell culture, pharmacological problems, and after transplant stem cell dispersion, remain in place. However, the challenge to comprehend how MSCs work remains to be the greatest challenge. Another challenge that arises is making MSCs more dependable and trustworthy to the average patient. The effectiveness of stem cell-directed differentiation must be increased (Zakrzewski et al., 2019). This further complicates the process. The long-term success of future stem cell treatments may be in question. The mass production of functioning, cooperative cells would be required to restore function to transplanted organs via stem cell treatment. Complicated processes are required to implement this kind of broad, ubiquitous regenerative medicine, which necessitates cooperation between medical professionals and others outside medicine (Zakrzewski et al., 2019). There has been a sturdy surge in the numerals of patients ready to undergo stem cell face treatments despite the many issues that have prevented it from being widely adopted. This research takes an in-depth look to uncover a comprehensive knowledge of the material and a correct course of action for facial jawbone renewal treatment using maxilla marrow stem cells.
Hypothesis
Inborn facial abnormalities are defects affecting the head and facial bones of a newborn. These disorders usually occur at birth (congenital) but vary in severity. Cleft edge and cleft palate are the most prevalent inborn disabilities. These craniofacial deformities require surgical operations to the damaged tissues. These operations are still widely provided through current therapy methods. Still, most contemporary treatments do not provide biological therapeutic results. Further improvements in cell and tissue-based methods are needed before the existing conventional therapies employing allogeneic and synthetic replacement treatments can fully overcome their current limits (Alérico et al., 2014)
Craniofacial applications typically utilize maxilla marrow where MSCs are extracted and used in cell-centered therapeutic strategies. Preclinical investigations have shown that autologous grafts containing bone marrow extracted cells may successfully treat craniofacial abnormalities, and many clinical trials are investigating their potential in bone regeneration. There are, however, certain drawbacks: cell separation methods are poor, and the grafts have not been described (Klijin et al., 2010; Bassetti et al., 2016; Le et al., 2018).
Chapter 2
Background
With about 2.2 million operations performed yearly globally, bone is among the most commonly transplanted tissues (Kinaci, Neuhaus & Ring, 2014). Autologous MSCs are collected from the patient and used to treat craniofacial defects. The procedure is presently considered the best in bone regeneration, providing a scaffold for the bone tissue to grow on (Sakkas et al., 2017). However, harvesting is a complex process with a significant drawback, as it requires a second surgical place, which yields only a small amount of bone marrow. In addition, the two-stage operation increases not only surgery time but also pain and damage of patient’s nerves during harvesting process. Also, the autologous bone resorption rate is variable. Increased medical expenses and patient suffering are all caused by these variables (Tang et al., 2016).
Additional surgery may be required in the maxillofacial area if one is suffering from a congenital deformity, serious face injuries, or if one has had surgery to remove a tumour. Teeth loss often causes bone abnormalities in the maxilla and mandible, leading to decreased horizontal and vertical dimensions in both the hard and soft alveolar regions (Le et al., 2018). Dental implants should be placed into a patient’s bone to have enough bone volume for implant success. Other surgical techniques have been devised to increase the alveolar crest’s (Alveolar Crest’s) dimensions (Black et al., 2015).
Animal, human, or synthetic bone replacements have been suggested as alternate techniques for bone regeneration, particularly due to the inherent problems with autologous grafting. Cases of infection are known to occur when bone replacement materials are used (Wolff et al., 2013). In addition, there are hazards of bacterial infection and rejection of the transplant from the recipient’s immune system to consider. Small bone defects may be repaired with these techniques, but bigger abnormalities are far less likely to be successfully fixed. There is currently a high clinical demand for both safe and effective treatments, which are danger free to patient (Ahmad et al., 2013; Thesleff et al., 2017)
Research on MSCs has increased dramatically in the last several years (Le, Rohrer & Prassad, 2010; Fekete et al., 2012; Zimmermann & Moghaddam, 2011; Kinaci, Neuhaus & Ring, 2014). These mesodermal stem cells are not hematopoietic and capable of multiple-lineage differentiation. There are many methods for extracting MSCs from various tissue types, including deciduous teeth, skeletal muscle umbilical cord, and fatty tissue. The majority of cell treatment uses MSCs from bone marrow, which have been in use for more than 30 years. Bone marrow and bone chips may be used to extract these cells (cortical or trabecular bone). these cells may promote bone formation if planted on or grown on calcium phosphate ceramic matrices. BCP is widely utilized for maxillary sinus floor reconstruction and extraction socket filling (Nkenke &Neukam, 2014; Brennan et al., 2014).
The recently completed preclinical research discovered that MSCs, in vitro and in vivo, may be cultivated on BCP ceramics, including 20% hydroxyapatite (HA) and 80% -TCP. This new clinical research used the maxillofacial area to see whether MSCs and BCP might help repair alveolar bone deficiencies. Several factors that contributed to this selection were the prevalence jaw trauma and deformities. Another critical problem in treating facial bone abnormalities is that they often can not be fixed, no matter how hard one tries. Currently, only allografts, autografts, or vascularized jawbone and soft flesh transplants derived from the same individual (in the event of a large, critical-sized lesion) can provide treatment alternatives for these kinds of problems. Secondly, the normal face architecture consists of a functioning dentition. While this is in place, the loss of teeth begins the continual resorption of the alveolar ridge (Kaigler et al., 2013). Denture wear typically causes significant bone loss and the loss of skeletal strength in the edentulous region. In addition, the restoration of oral function remains a problem due to the minuscule remaining bone volume and the deteriorating rate of resorption. For patients who do, implant placement is critical for restoring oral function, and therapeutic choices are restricted. Ethically, it is now permissible to biopsy the implant site after implant installation since the technique makes it possible to evaluate the integrity of freshly produced bone (Hanson, 2012).
A new bone augmentation strategy was used in the current clinical study in humans It was essential to test and implement the novel method that utilizes MSCs for medical trials to demonstrate the effectiveness, safeness, and practicality of the innovative technique. This technique included the harvesting of cells, which were then grown for three weeks before being transplanted to the locations of defects. The alternative result was to place alveolar implants in the increased dental bone and use fasten retention on a fixed partial dent that was then screwed into the implants (Raynaud, & Rafii, 2013).
Methodology.
This research follows worldwide standards for advanced therapeutic pharmaceutical goods, which means it fits all of the required approvals. Two physicians who have significant expertise with the research instructed the patients about it. Clinical photos and dental imprints were taken after permission had been signed, after which the patients had a clinical examination, including medical history, performed on them. Thirteen people volunteered to participate in this clinical research. All of them were patients at the maxillofacial surgery Section. The eligibility of the patients was to be medically fit, non-smokers, have blood tests resulting to no traceable of communicable diseases, and aged between 19 and 70 years. Additionally, this set of specifications required a dental missing tooth, and a dental ridge width in the mandibular posterior region measuring less than 4.5 mm, and have at least two teeth in the maxillary anterior region. Before any study-related intervention, all participants gave signed informed permission. Seventeen people participated in the experiment, and their bone marrow samples were collected from the subsequent iliac peak while they were under anaesthetic. Every bone marrow sample was extracted in small aliquots divided into two to four portions and placed into 20 ml syringes and capped with a Luer lock connector. All operations were done in a grade A cleanroom under laminar hood flow. As previously explained, the expansion was carried out as planned.
Clinical procedures
The doctor did everything while under local anaesthetic, and all the operations were done by a surgeon who was well-trained. CBCT radiographs were obtained for each patient to assess the volume of the bone before grafting (T0) and later another one after 4–6 months after grafting, (T1). A dose of 1 g of amoxicillin was given one hour before to surgery to all patients. The region was anaesthetized with local anaesthetic prior to surgery. In order to improve blood movement and promote muscle ingrowth into the biomaterials. A fold was elevated, and the jawbone was pierced with a tiny round burr (Fig. 2A). To give the affected limb some semblance of balance, a lightweight titanium-reinforced non resorbable PTFE) membrane was affixed to the jaw bone using sizable screws used to create a tent-like appearance.
5 cm3 of BCP in the form of granules packaged in syringes were given to each patient, and 100 million MSCs were injected into the mixture with the BCP in the form of granules during surgery. The second stage (proximal migration) took place over the next 60 minutes, during which MSCs connected to the BCP granules in the syringes. In order To produce a final dosage of 20 × 106 cells/1 cm3, the number of cells had to be combined with BCP. The MSC-laden BCP granules were removed from the syringe and administered through an injection (Fig.2B). Bacteriological assays and cell attachment were also conducted using the remaining mixture. The DNA binding dye DAPI was employed for cell attachment. In order to create a pocket around the bone and regenerative membrane, a cell-seeded substance was injected into the opening between the bone and regenerative membrane, and then the opening was closed with the film and mucoperiosteum flaps (Fig. 2C). The final closure was done using nonabsorbable sutures to close the outer ears.
After 10 to 14 days, patients were advised to consume only soft foods, and they were also encouraged to use chlorhexidine mouthwash every day. Antibiotics were administered for seven days. In order to relieve pain, paracetamol or codeine phosphate sesquihydrate was given by mouth.
The surgeon checked the patient clinically at the operation site and removed the sutures 12 days following the surgery. Aspects of the patient’s anatomy were scanned using an image-guided approach (T1). To conduct follow-up exams, patients were re-contacted after a period of 4 months (Fig. 2D). Four to six months after surgery, CBCT scans were performed to see whether the locations were ready for implant placement.
Even if the breadth of the enlarged region was 7 mm or greater, it was re-entered during implant placement (Fig. 2E). Before implant placement, bone samples were obtained under local anaesthetic. The results were examined using histology and micro-computed tomography to see whether new bone growth was taking place. After performing all of the necessary checks, the manufacturer’s instructions were followed, and dental implants with a specified diameter and lengthwere then placed (Fig. 2F). About two months following implant placement, the abutment surgery was done, and then a screw-retained crown was placed (Fig. 2G) (Fig. 2H). These operations each had the graft stability quotient evaluated at them.
Outcomes
The main results of the trial were the study’s ability to safely and successfully complete the operation after a year. To gauge the safety of a system, an incident reporting procedure was created. These occurrences are categorized as either severe adverse events or serious adverse responses based on guidelines from the international Medical Agencies. Examples of adverse effects, include local (such as, infection or hematomas), systemic, (such as fever or anaphylaxis). The technique’s practicality was assessed based on two variables: the capacity to manipulate the graft surgically and the ability to perform the implantation operation as intended.
Results
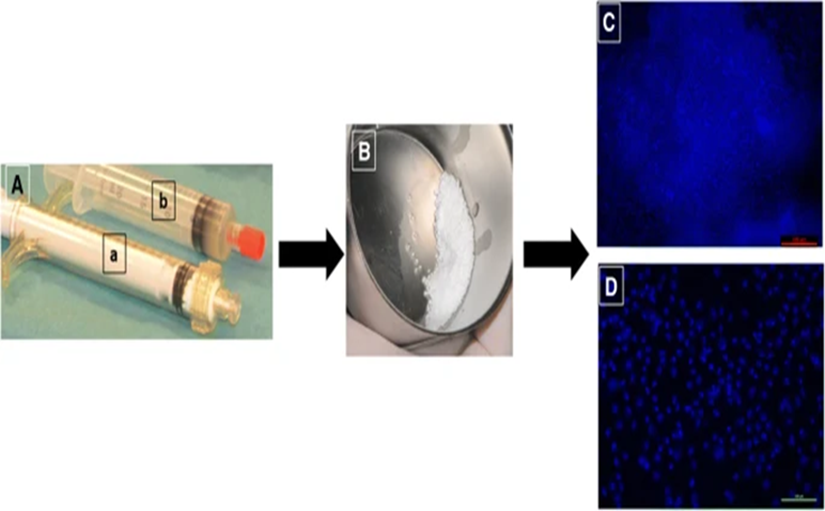
The final cell product was 90% viable and comprised freshly obtained autologous MSCs grown in vitro and then stably transfected with the markers CD90, CD73, and CD105, all of which were adverse for both CD14 and CD45. The results for CD49d, CD90, and CD105 were notable, with substantial inclusion in CD14 and CD106. The vitality of the cells upon coming to the operation room was between 87% and 90%. The surgical mixing was done in a controlled environment under aseptic circumstances (Fig. 1A, B). The BCP granules were evenly incorporated into the culture after 60 minutes (Fig. 1c and d).
(A) Plungers carrying BCP pellets (a) and MSC cells (b). (B) BCP and MSCs are combined. Following arrival in the operating room, DAPI staining is used to assess cell attachment to the biomaterial (c) and (d).
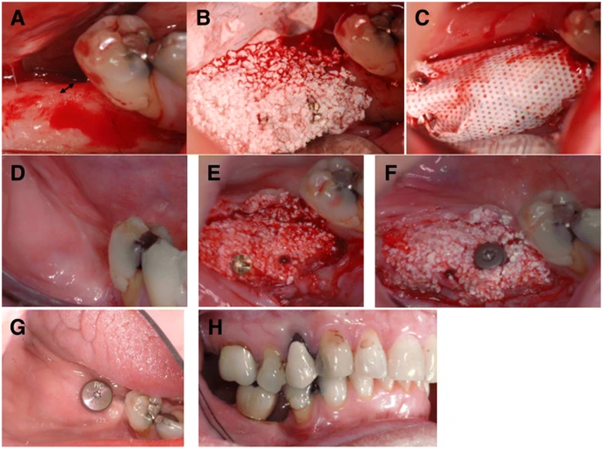
Figure 2 Clinical operation process.
(a) Prior to augmentation, the alveolar ridge was narrow (arrow). (b) Alveolar ridge was treated with a mixture of BCP and MSCs. (c) Placement of a membrane over the transplanted graft. (d) After 5 months, soft tissue healing has occurred. (e) After 5 months after healing, a new alveolar ridge has formed. (f) A core biopsy is performed, and the dental implant is placed in the freshly formed jawbone. (g) Eight months after the expansion practice and two months after the grafting practice. (h) Occlusion of transplant reinforced top
Thirteen patients whose age ranged from 52 to 69 years (mean age 55.23 years) were enrolled. Every patient met the expansions’ requirements, and cells could be given to all of them. Due to the lack of MSCs in the starting material, the expansions had to be halted at passage. During the study period, no adverse effects occurred. Also, the supplemented bone had a more significant amount of keratinized gingiva, and the softer tissues that covered it had a strong soft material profile (Fig. 2d). In the end, jawbone development was significantly affected by the membrane’s location.
Table 1 Development of cells taken from 13 individuals’ bone marrow.
(x)- No collection making unit fibroblast growth. (y)- Inadequate cell tally.
| Patient number | BMSCs/μl BM number of MNCs | BMSCs/μl BM in passage 1 | Overall produce culture passage 1 |
| 1 | 32500 | 29800 | 305000000 |
| 2 | 21300 | 152000 | 419000000 |
| 3 | 35800 | 28900 | 247800000 |
| 4 | 1730 | 244000 | 4037000000 |
| 5 | 47.4 | x | y |
| 6 | 56100 | 62700 | 4122000000 |
| 7 | 4910 | 5270 | 597300000 |
| 8 | 17100 | 2.26 | 2896000000 |
| 9 | 25400 | 16700 | 166000000 |
| 10 | nil | x | y |
| 11 | 56500 | 76700 | 582000000 |
| 12 | 360000 | 28500 | 399000000 |
| 13 | 4540 | 27900 | 2493000000 |
| sum | 615927.4 | 672472.26 | 16264100000 |
| mean | 47379.03077 | 61133.84182 | 1478554545 |
| SD | 99133.72267 | 74137.66933 | 1582877069 |
Ridge augmentation was done on all 11 patients, and sufficient bone was available for dental implant placement (Table 2). No issues were encountered during the PTFE membrane replacement, seven to eight weeks after augmentation.
| Patient number | Age (years) | Sex | Healing period (weeks) | Growth in breadth (mm) | Growth in size (mm3) | Graft placement | Head delivered | Patient satisfied |
| 1 | 55 | Male | 25 | 4.9 | 1298.34 | y | y | satisfied |
| 2 | 67 | Female | 30 | 7.6 | 1983.22 | y | y | satisfied |
| 3 | 55 | Male | 28 | 4.5 | 1230.66 | y | y | satisfied |
| 4 | 52 | Male | 28 | 2.2 | 2010.11 | y | y | satisfied |
| 5 | 62 | Female | 27 | 3.8 | 1379.22 | y | y | satisfied |
| 6 (left) | 59 | Female | 21 | 5.4 | 1427.9 | y | y | satisfied |
| 7 (right) | 62 | Female | 25 | 3.5 | 1235.77 | y | y | satisfied |
| 8 | 65 | Female | 28 | 2.5 | 623.45 | y | y | satisfied |
| 9 | 61 | Male | 32 | 3.5 | 499.23 | y | y | satisfied |
| 10 | 56 | Male | 31 | 7.6 | 2147.87 | y | y | satisfied |
| 11 | 55 | Male | 29 | 3.8 | 845.29 | y | y | satisfied |
| 12 | 69 | Male | 26 | 4.1 | 516.78 | y | y | satisfied |
| 13 | 60 | Male | 25 | 4.6 | 735.96 | y | y | satisfied |
Proves bone therapeutic, increased bone volume and width where, every patient was grafted and prostheses with (y) yes.
A linear measuring process was implemented to determine the width and height of all scans using the scanning software. The scans immediately after the grafting process were compared to each other to determine their density and structure. The operation was easy to discern between the grafted bone and residual bone. When doing measures that are reliant on the operator, the measurements were done by radiology expert, whose specialization is oral radiology (SS). The patients’ alveolar walls had increased enough to allow implants to be placed (Fig. 4 and Table 3). 887.23 ± 365.01 mm3 (Table 3). Alveolar ridge width increased, as did alveolar ridge volume. Mean bone width increased by an average of 4.05 mm, with a 95% confidence interval ranging from 2.74 to 5.36, whereas mean bone volume increased by an average of 887.23 mm3, with a 95% confidence interval ranging from 676 to 1097.98.
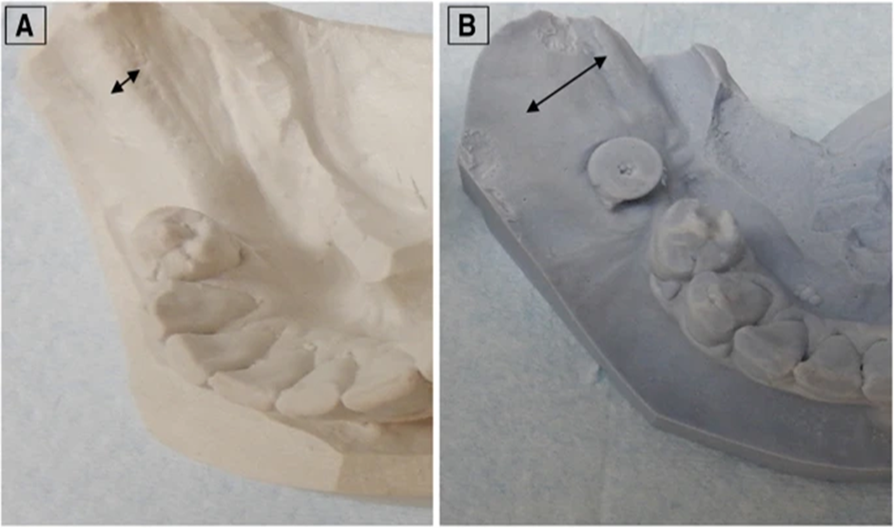
Figure 3 Alveolar ridge cast. showing the quantity of bone rebuilt. The arrows show the alveolar ridge’s breadth before and after augmentation respectively.
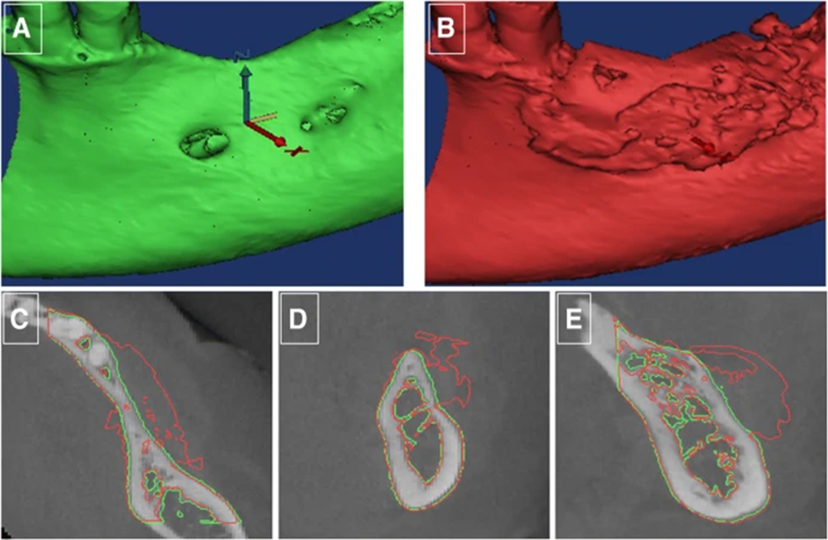
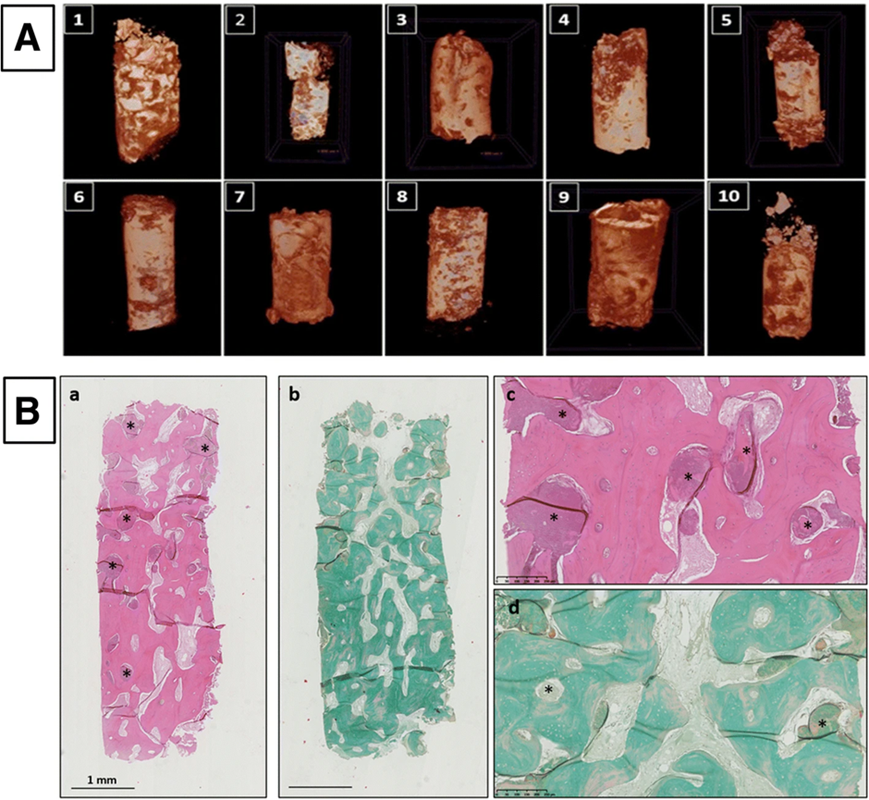
Overlapping bone shape ridges of overlaid models before transplanting, green (T0) and after transplanting, (six months, red, T1) were obtained and seen 3 dimensional radiographic views (in axial, sagittal and coronal respectively) of the region before and after regeneration.New bone tissue, consisting of osteoblast inside layer cells and lamellar bone tissue, was shown to have BCP granules completely embedded inside the particles. (Fig. 5B). When BCP granules were studied, it was found that these products had substantial disintegration and dissolution; along with the appearance of multinucleated osteoclasts, CD68-expressing macrophages, and osteoclast-like cells, they also could float.
Figure 5 CT scans and histopathological examinations.
- A) CT pictures of biopsies taken from 10 patients are shown. (B) interpretation of the biopsy results of the samples obtained from patients. These overlapping, intertwined lamellar bones with entangled osteocytes that form the extracellular matrix around the remaining BCP particles are evident when the specimen is magnified at high power. Hematoxylin and eosin staining in (a) and (c). Stained with Masson trichrome in (b) and (d) under mmagnifications of 1.25 and 10 times.
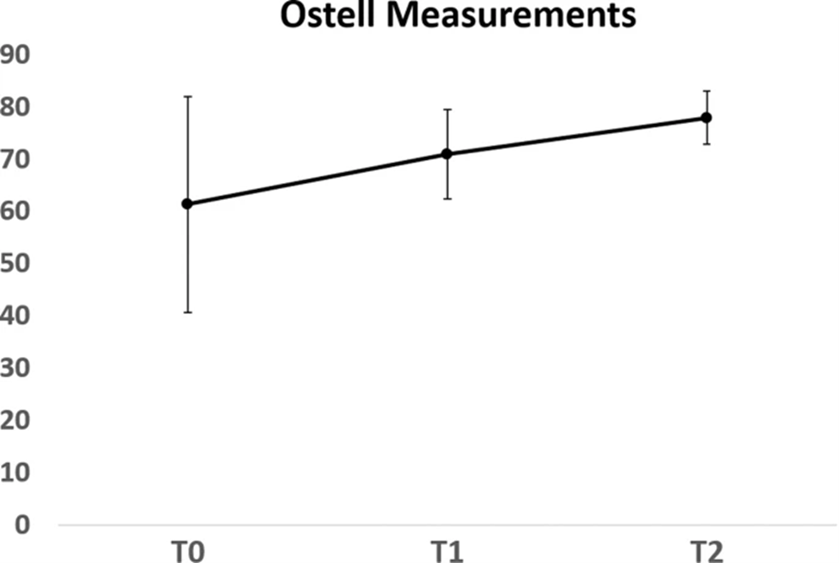
Patients were pleased with both the cosmetic and functional results and the absence of any side effects. All of the transplants and the donor location had no postoperative infections. When the membrane is exposed, the patient experienced moderate degrees of discomfort. After surgery, the other patients reported very mild discomfort. The clinical result of the augmentation operation was well-received by all patients, and they were equally satisfied with the new dental set. Patients promised to encourage others with a comparable clinical situation to have this treatment. Ostell levels increased in all of the individuals who received dental implants over the first 12 months following implantation.. Fig. 6.
Increased in implant stability was seen in the graph provided as mean ± SD, demonstrating that implants load up well. T0: implantation, T1; loading, and follow-up at 18 months (T2).
Discussion
In this clinical research, all individuals in the study had augmentation of alveolar bone due to utilizing bone marrow-derived MSCs. The posterior mandibular ridge was the location chosen for bone augmentation. Reconstruction in this location is challenging because of a bit of blood supply, the contaminated environment, and many oral functions, which affect the implant’s capacity to remain stable. Despite these many difficulties, we were able to achieve new bone growth and augment the alveolar ridge.
The results show that vertical alveolar ridge augmentation is not predicted, whereas horizontal alveolar ridge augmentation is (Janssen et al., 2017; Faia-Torres et al., 2015). Uncertainty about whether the graft will rot out or grow depends on factors such as the donor’s health, the quantity of bone that can be removed, and whether or not the graft will be resorbed (Atieh et al., 2015; Faia-Torres et al., 2015). Both horizontal and vertical growth was enhanced using the stem cell/biomaterial combination in the current study. Patients reported little of the donor site morbidity, the researchers found. What made this method unique was the creation of a viable procedure for the production of clinical-grade cells. Both MSC expansion methods (non-osteogenic) and cell expansion methods (using osteogenic factors) did not utilize osteogenic factors. Another possible reason of the increase in cell production costs is that growth agents may have diverse effects on tissues, and that may cause the price of cell creation to rise.
MSCs were generated in a bioreactor in the same manner that they would be in this clinical study. In other animal models, new bone was formed in conjunction with the BCP biomaterial, and this was shown by shipping cells within 24 hours and applying the treatment fresh. The study has connected it to a particular cell to biomaterial ratio or a specific amount of cells for bone formation.. Results from preclinical studies where 20 million MSCs were mixed with one cubic centimetre of BCP were used in clinical study (Brennan et al., 2014). . This suggests that MSCs’ innate ability to create bone validates the study since they were not altered. As stated earlier, MSC differentiation may be assisted by PL throughout the isolation and expansion stages, but the impact of this on osteogenic differentiation has been disputed. Recent research determined that using up to 80 different donors reduces the variance in factors for growing, cytokines, and chemokines ensuring stable conditions for ex-vivo MSC development.
Due to differences in MSCs concentrations in bone marrow aspirates, two patients had inadequate cell growth in vitro. However, by increasing the number of collected or developing cells to identify the relevant cells before starting culture, variability may be addressed. Research on jawbone marrow and adipose-derived stem cell restoration of mandibular and maxillary defects are few; nevertheless, many of these publications are case reports (Wolff et al., 2013; Rajan et al., 2014; Sándor et al., 2013;). Published research differs with the kind of cell used, defect location, material, the number of cells, the use of growth agents, and the presence of membranes or hardware. However, the current evidence was derived by treating eleven patients without any growth factors or stimulants utilized beforehand. As an inclusion criterion, since bone repair relies on the location of the defective bone, all patients had the posterior mandibular area chosen. The barrier also complicates surgical procedures, such as suturing and healing procedures, such as dressing changes. A high-density membrane is made of a microporous material, resistant to germs, but permits the passage of gases and tiny molecules, and oxygen from the periostea, which reduces the blood stream to the implant and limits vascularization. The pellets outside the lining which persisted, did not promote bone development, showing that the correct membrane is critical in obtaining bone growth. In an additional study Rajan et al., (2014) found that the membrane was essential to bone formation, and failed to produce bone formation when ceramic bone substitutes and MSCs were cultivated in osteogenic media for seven days with no membrane and no bone marrow.
A newly published randomized, controlled study found that bone marrow-derived cells placed on gelatine sponges effectively repaired osseous abnormalities created following tooth extraction. After six weeks, the defects began to mend more quickly, but at 12 weeks, no difference was seen between the group that had no cells put into the defect and the control group Many know that extraction sockets heal on their own, with no treatment required.
In the current research, visual assessment of volume on CBCT images was used to evaluate the effectiveness of a graft. Bone volume changes at T0 and T1 were volumetric. In order to validate the clinically reported volumetric changes in the graft, a standardized assessment was done using CBCT pictures. Grafting operations performed in patients with alveolar clefts have also utilized this approach in follow-up care (Xiao et al., 2016; Janssen et al., 2017). More importantly, after four to six months after implantation, the biopsy results revealed substantial new bone growth, with a generous blood supply and devoid of inflammatory cells. The reported resorption period for the BCP scaffold is between two and two and a half years. Extracellular support and stimulates of the cells are provided by the scaffold material, which also delivers the cells. The claim may be made that at least some grafted cells were involved in bone regeneration since the central area of the defect and implant site were used to get the bone core specimen.
This resorbed keratinized mucosa does not renew. It is advantageous to have a 1–2 mm layer of keratinized mucosa around an implant since this helps to reduce plaque build-up, tissue irritation, and the rate of attachment loss. Additionally, augmentation opened up keratinized mucosa, which was previously unknown (Fig. 2d, g). Cells used to repair bone have an apparent beneficial impact on soft tissues even when encapsulated, it seems. There is preliminary evidence that MSCs have a positive impact on wound healing (Fujio et al., 2017). This finding calls for additional study. However, paracrine signalling seems to be the mechanism responsible for MSCs wound-healing effects. As research has shown, paracrine substances generated by stem cells are thought to encourage angiogenesis and osteogenesis (Raynaud & Rafii, 2013; Fujio et al., 2017) IGF-1, VEGF, and TGF-β1 mediate this paracrine effect. In addition to activating the cell cycle, researchers found that growth factors may help stimulate cell production, migration, the formation and differentiation of blood vessels.
Due to the limited sample size and follow-up duration to three years, these results should be viewed with caution. This treatment regimen requires more study to be used in a clinical setting. A greater sample size and a longer duration of follow-up are needed. While this discovery is still in the experimental stages, it does have the potential to yield regenerative medicine and new therapeutic interventions, which in turn will affect a broad range of patients. . (Lin, Chan & Wang, 2013; Osugi et al., 2012)
Summary
Every year a significant number of bone transplants are needed to treat individuals with severe bone fractures. Due to their inherent restrictions, autografts and allografts could not be used in clinical settings. Another approach is to utilize tissue engineering techniques to provide new options for bone transplants for clinical purposes. Much progress has been made in bone tissue engineering during the last two decades, but translating this advancement into practical applications is still a significant problem. These non-hematopoietic BM-derived MSCs may develop into a group of mesenchymal cells, which can together be referred to as a “plastic-adherent group.” This cell type has been shown to give rise to many mesenchymal and non-mesenchymal lineages, which have been investigated regarding their role in the creation of the marrow microenvironment. Since they are able to change into many cell types, MSCs are an excellent cellular source for cellular treatment. A small number of cell markers, including those that lack CD31, CD34, and CD45 and possess CD13, CD29, CD73, CD90, -, CD105, and CD166, MSCs may be phenotypically recognized. To understand the formation of adipocytes from precursor stem cells producing bone and the relation to or characterized by chondrogenesis lineages, in vitro, MSCs differentiate according to the medium, the culture conditions, and the cell preparations. Clinical repair of the dental ridge using MSCs and BCP derived from the same individual was shown to be feasible, safe, and predictable in this clinical study of human patients. To the best of our knowledge, all of the implants had been successfully augmented. All implants placed using a screw-retained dental crown were integrated, with the crown screw anchoring the implant to the jawbone. The augmentation method proposed here has so far shown itself effectively, and therefore should be followed up with further investigation and perhaps even provide the basis for a proper treatment plan that may be utilized to challenge the current standard in the area of aesthetic medicine.
Conclusion
In this new clinical investigation in human patients, the researchers discovered that medical restoration of the dental jawbone using extracted MSCs and BCP is possible, benign, and predictable. All places were properly increased, and all dental scions were successfully Osseo-integrated and repaired with screw to retain dental heads, just as the doctors had envisioned. Therefore, this new augmentation technique merits further study and may serve as the foundation for a legitimate treatment regimen that would be a viable alternative to the existing gold standard. Although regenerative medicine has been attempted in various areas, there is a high need for it in dentistry, especially in bone regeneration. Occlusal reconstitution may take anywhere from 6 to 12 months, depending on the severity of the periodontitis or the extent of the jaw resection. As a result, developing a bone derivation technique that is both efficient and of excellent quality is required. Cell-based therapy may open the way for regenerative treatments for bone abnormalities that are not subject to rejection. Research on growth factors or cell-based treatments is also expected to continue to develop in the foreseeable future. Despite this, many issues must be addressed, such as legislation and the price of acquiring equipment. Even though it is unknown when regenerative medicine technology will be put into practical use, it is essential to keep up with the present state of regenerative medicine to stay on top of its development.