Because creating a solid research design is the first significant step in producing a research paper, you must understand how to do so. A research design will act as the basis or framework for your article; your study will be stalled without it. It is the foundation of your research paper and the component that will assist you in answering your research questions. It comprises methods and procedures for doing research and combining its many components, which you must describe and demonstrate.
Follow the steps below if you want to learn how to build a good study design.
Steps to Putting Up a Solid Research Plan
The stages for developing a good research design are outlined below.
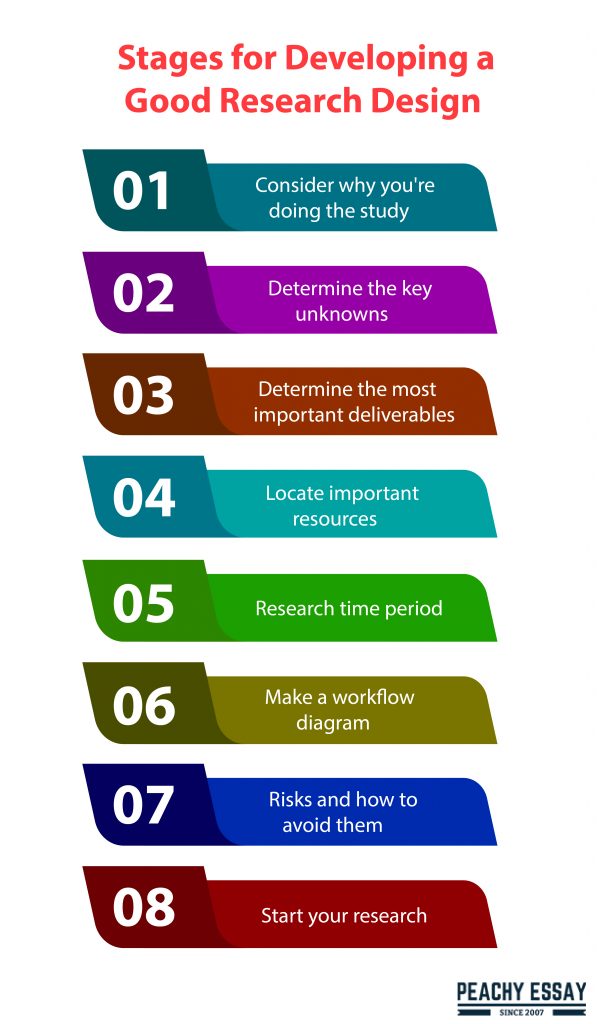
Consider why you’re doing the study
The first step in your study design is to figure out what you’re going to conduct and why you’re going to do it. Many individuals get so engrossed in their endeavor that they lose sight of the forest for the trees and believe that everyone understands the significance of their effort. This is not the case; you must convey the significance of your work to non-experts. Your examiner will be checking to determine whether you comprehend the significance. Start with a reason, whether you’re writing a proposal or an introduction to your thesis.
Grants are given out competitively based on their significance and relevance. Only relevant papers are published. The reason is arguably one of the essential elements of your study design, and you’ll be wasting your time if you skim over it or disregard it.
Determine the key unknowns
It would be best if you studied upon prior work after settling on the broad topic of your research subject and developed a solid justification and purpose for doing it. What are the most significant unknowns and research questions? What gaps does my research project have the potential to fill? Write a “Wider Justification” in which you examine prior work while also identifying knowledge gaps.
If you’re writing a Ph.D. or MSc dissertation, you should plan to spend a significant amount of time reviewing the existing literature at the outset of your research endeavor. As you come across research topics and critical unknowns, make a list of them. This is a crucial step in establishing yourself as an authority in your field.
Finally, double-check that your research hasn’t already been done at this point!
What is your purpose, and what are your goals?
After you’ve established a justification for your study, you’ll need to choose a goal. This is the most crucial aspect of your research plan, and it should cover the major unknowns outlined in Step 1 above. You should be able to explain your goal in one phrase at the most.
Your goals should assist you in achieving your goal. You should establish 3-5 goals that will each help you get closer to your goal. Ideally, each goal should be linked to a set of research questions so that you’re constantly striving to come up with something new and unique. This will also help to keep your study focused and on track.
The following are common qualities of good goals and objectives:
- Specific, attainable, and doable
- A clear understanding of the deliverables
- The overarching research question that is specific, precise, and concise
- In terms of techniques and timeframes, be realistic.
- Compare, describe, explain, quantify, interpret, and measure are examples of words to use
What theories are you putting to the test?
Hypotheses must be tested scientifically. Your study of past work and important unknowns should help you identify these possibilities. A competent scientist should try to disprove her own ideas. Your theories should be founded on the literature and your identification of the important knowns and unknowns, and you should advance science.
Determine the most important deliverables
What will your research’s primary outcomes and deliverables be? Understanding, quantification, conceptual, process, analysis, characterization, and determination should all be included in the deliverables. Consider the following scenario:
These deliverables should allow you to put your theories to the test and accomplish your goal. They should be precise and attainable, and they should assist you in answering your “Big Question.”
Locate important resources
What are the resources you’ll need to do this research project? Will you have to conduct fieldwork, and if so, how long will it take? Are there any particular computer resources, packages, applications, remotely sensed pictures, or computer codes that you’ll need?
Research time period
A realistic evaluation of the time requirements for each goal should be part of your study plan. Make a Gantt Chart outlining each goal and the amount of time you have available (you can do this in Excel or on paper!). Calculate how much time each goal will take you in detail, and be realistic about whether you can do it in the time you have available. Students often underestimate how long it will take them to complete a task, so be conservative in your estimates.
Make a workflow diagram
After you’ve completed the stages above, you’ll be able to bring it all together into a logical workflow model. These, in my opinion, should be included in all dissertations and grant proposals since they clearly show how the various goals relate to one another.
Write the goal at the top of the page, followed by the hypotheses. Below here, list your resources or inputs. Then, in a separate box, write down each goal and the important deliverables that go along with it. Finally, at the bottom, state your outcome, such as whether your hypotheses were accepted or rejected. The process model should show your deliverables and therefore bring your ‘Biq Question’ to a close.
Risks and how to avoid them
If you’re going to undertake fieldwork, you’ll need to do a risk assessment and explicitly define potential dangers, as well as how you’ll minimize or avoid them. However, you should be aware of broader dangers; do you have the necessary knowledge? Are the materials you need readily available? Will the prices fluctuate? The following are some potential dangers:
- Unreliable exchange rates
- Wildlife dangers
- Weather
- Hazards to the environment and garbage disposal
- Failure of the equipment
Start your research
You’re ready to start your research now that you’ve spent some time properly preparing it. You will research a topic that is relevant, interesting, and enjoyable to you. You’ve developed a solid study proposal and are certain that your findings will be useful to society and other scientists.
Evaluate your work as you go, and be willing to alter your approach or goals if you discover something isn’t possible or too tough. In fact, if your techniques don’t work or it turns out to be impossible, your effort may need to go full circle!
Elements of a Research Design
The research design will be determined by the kind of research issue that a business is experiencing, not the other way around. The study’s design phase decides which tools to employ and how they should be utilized.
Effective research reduces data bias and improves confidence in the veracity of gathered data. In experimental research, a design that provides the smallest error margin is usually the desired result. The following are the key elements:
- Statement of intent that is accurate
- Techniques to be used in the collection and analysis of data
- The technique used to examine the information gathered
- The methodology used in research
- Potential research stumbling blocks
- The research study’s environment
- Timeline
- Analytical measurement
Your study will be more successful if you use the right research design. Successful research projects offer reliable and impartial information.
Characteristics of Research Design
The features of the research design are as follows.
-
Neutrality
You may have to make assumptions about the data you anticipate gathering while planning your research. The study findings should be impartial and devoid of prejudice. Consider those who agree with the obtained findings and get various views on the final assessed scores and conclusions.
-
Reliability
When researching regularly, the researcher wants consistent findings. To guarantee the quality of your findings, your design should show how to formulate research questions. Only a dependable design will allow you to get the desired outcomes.
-
Validity
A variety of measurement instruments are available. The only proper measuring instruments, on the other hand, are those that assist a researcher in gauging findings by the study goal. The questionnaire created as a result of this design will be legitimate.
-
Generalization
Your design’s result should be generalizable to the whole population, not simply a small sample. A generic design means that your survey may be performed with equal accuracy on any segment of the population.
Research Design Classification
To choose which model to use for a study, a researcher needs to understand the different kinds of research design thoroughly. The design of your study, like the research itself, may be divided into quantitative and qualitative categories.
Qualitative
Qualitative research uses quantitative computations to identify connections between gathered data and observations. Statistical techniques may be used to verify or refute theories about naturally occurring phenomena. Researchers use qualitative research techniques to determine “why” a certain theory exists as well as “what” respondents have to say about it.
Quantitative
Quantitative research is used in situations when statistical findings are required to get practical information. Numbers provide you a greater perspective when it comes to making important business choices. Quantitative research techniques are required for every organization’s development. When it comes to making business choices, insights derived from hard numerical data and analysis have shown to be very useful.
Types of Research Designs
Various research strategies are suitable for different circumstances and research topics. To put it another way, each design style is best suited to a particular function. A case report, for example, may be the best option for investigating a novel disease that has never been observed before, while a randomized controlled trial would be inappropriate.
The many kinds of study designs are listed below.
Case reports and case series
Case reports are published accounts of an individual patient or incident or a small number of patients or occurrences that show distinctive features of interest in health research. Typically, the reporter will explain ordinary characteristics, such as a combination of presenting signs and symptoms, illness sequelae or trajectory, or unanticipated results, using a thorough and detailed evaluation of the person.
Case reports and case series may be used to spot new trends or illnesses, discover medication side effects or novel applications, exchange rare event experiences, and explain unusual medical occurrences. These investigations are essential because they may lead to the emergence of new problems or concepts and the generation of hypotheses for future investigation.
They may not be generalizable, may wrongly assign cause and effect, and therefore be misleading. They are not based on rigorous research techniques since they are single occurrences or a short sequence of events. Rather, the case report as a whole serves as a point of reference for a particular observation that notifies a community of practice and may then be recorded for future research and comparison. The case series data may be utilized to create a knowledge base and generate hypotheses that can be evaluated in more rigorous research designs.
Cross-sectional approach
Let’s define the word prevalence before moving on to cross-sectional research. The frequency of a condition of interest assessed at a certain time is referred to as prevalence. Because it estimates the number of instances within a sample collected at a certain moment in time, the prevalence score, or prevalence estimate, is also known as the point-prevalence rate. The prevalence estimate takes on a rate value compared to the total number of people at risk in the sample.
Given this prevalence definition, we can conclude that prevalence may be studied using a cross-sectional research method. When measurements are taken just once across a sample in cross-sectional research, the prevalence rate is often employed. If the sample used to estimate is genuinely representative of the population from which it was taken, prevalence rates may be deemed generalizable to a wider group.
Because data is collected in a single bout (one-time sampling), the cross-sectional study design has fewer expenses than a longitudinal research project.
However, one disadvantage of one-time sampling is that certain people who may impact the study’s result may be overlooked during the sampling phase. Similarly, since the sample is not followed up on and frequently has no history information, virtually little can be drawn about causal connections from the sample.
Longitudinal research
Longitudinal research is a kind of observational research. The longitudinal study, in contrast to the cross-sectional study, which measures variables in a sample of participants at a single point in time, and the case-control study design, which measures the outcome first and the risk factors after the outcome has occurred, measures variables in a sample of participants over time. In addition, since the longitudinal study is observational, the researchers cannot interfere or impose any stimuli on the subjects.
Nonetheless, since the study is longitudinal, the researcher may gather data according to an apriori measurement plan and assess results at the individual level. Time-lagged, pre-post, and repeated measurements designs are all terms used to describe longitudinal research designs. It’s worth noting that data from at least three time periods must be gathered for research to be genuinely longitudinal.
The incidence rate is often an essential estimate in longitudinal research (also called the incidence density rate). The incidence rate is calculated by dividing the number of new cases detected during a specific time period by the population at risk during that time period. Calculating the incidence rate allows researchers to evaluate the effects of a particular treatment regimen, monitor prescription adherence, measure the impacts of aging, and verify policy compliance.
Cohort studies
Cohort studies are one of the most popular longitudinal research designs. An observational study in which the researcher merely watches a result without interfering is a cohort study. Cohort studies track a group of people with comparable features either in the future (prospectively) or in the past (retrospectively) (retrospectively). Incidence rates are studied using cohort research methods. In a cohort study, the group that exhibits the desired characteristic(s) is tracked for a long time and compared against a comparable group that does not exhibit the desired characteristic(s). The chosen group’s specific metrics are compared to those provided for the comparative cohort. During the monitoring stage, measurements are usually collected at the start of monitoring, at pre-determined times during the research, and then at the end.
Experimental research designs
In its most basic form, an experiment is an evaluative process in which the researcher controls the circumstances that are applied to a certain set of participants and observes the occurrence of a specific result. Consider an experiment to see how resting systolic blood pressure changes are affected by taking a medication vs. placebo.
In step one, the researcher chooses a group of people at random from the population. Individuals in the group are then assigned to one of two groups: the medication group or the placebo group, at random. The participants in both groups’ resting systolic blood pressure are measured in step 2. Individuals in the drug group are given a tablet to lower blood pressure in step three. Individuals in the placebo group are given a tablet with a similar form that is a placebo. In step 4, participants in both groups wait one hour before having their resting systolic blood pressure checked again. Two methods are used to compare the differences in systolic blood pressure readings.
First, the medication group’s average change in blood pressure is compared to the placebo group’s average change in blood pressure. Following that, the average pre-blood pressure in the medication group is compared to the average post-blood pressure in the placebo group. The following pictures depict the sequence of events in this basic experimental design


