Abstract:
The efficacy of three Taify rose-endophytic fungal filtrates at different concentrations on juvenile mortality and egg hatching of Meloidogyne javanica after different exposure times was evaluated in vitro. The results revealed that exposition to culture filtrate of Penicillium citrinum isolates MN518391 at 8% for 24 h significantly gave the highest reduction in the numbers of viable M. javanica J2s and hatched eggs. The characteristic appearances of the nematode J2s incubated for 24 h in the highest concentration of the fungal filtrates clear that most nematodes exposed to P. citrinum characterized with straight body shape, implying reduction in viability, whereas it mostly exhibited the bent shape after exposing either to Aspergillus niger MK713445 or A. niger MN513383 filtrates. GC-MS analysis of the three fungal filtrates detected 22 chemical constituents that may be responsible for nematicidal properties. Of which, 6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline compound was found in a major amount, whereas squalane was a less predominant compound. Our study proved that the three endophytic fungi isolated from Taify rose could be used for biological control of root-knot nematodes.
KEYWORDS
Biological control, Endophytic fungi, Root-knot nematode, Meloidogyne javanica, Taify rose
INTRODUCTION:
Root-knot nematodes (RKNs), Meloidogyne spp. are pests of economic importance found worldwide and have been reported on different plant species, causing quality and yield crop losses up to 80% in susceptible plant species vegetable plants (Kaskavalci, 2007). In Saudi Arabia, the RKNs are widely distributed throughout different agricultural areas throughout the country, with M. incognita and M. javanica reported as the most common species parasitizing roses (Al-Hazmi et al., 1983; Nour El-Deen et al., 2015). When Meloidogyne spp. infests plants, the normal root system is reduced to a low number of extreme galled roots with a fully disorganized vascular system. Rootlets are almost lacking. The roots are strongly obstructed in the uptake and transport of water and nutrients (Netscher and Sikora, 1990). Most effective nematicides used to control RKN are very expensive and have negative environmental impacts. In the last decades, bio-control agents received greater attention for nematode management. Antagonistic fungi have been successfully used for nematode control without causing environmental hazards (Holgado and Crump, 2003; Chen and Dickson, 2004; Lopez-Liorca and Jansson, 2006; Mukhtar, 2018). Devi and Bora (2018) recorded that culture filtrates of Trichoderma viride, T. harzianum, Trichoderma sp., Fusarium sp., Penicillium sp. and Aspergillus sp. significantly induced larval mortality and egg hatching inhibition of Meloidogyne incognita race 2. Several compounds with nematicidal activity have been documented from fungi, but no major commercial product based on these natural fungal compounds has been produced for wide use (Li et al., 2007; Anke, 2010). Using fungal endophytes against phytonematodes has previously been studied (Zabalgogeazcoa, 2008). There is evidence that endophytes may affect nematodes either directly by synthesizing nematicidal compounds which kill or paralyze nematodes or indirectly by encouraging plant defense toward nematode (Schouten, 2016). The endophytic fungus, Acremonium implicatum isolated from galled tomato roots has excellent potential for M. incognita control (Tian et al., 2014). Yan et al. (2011) found that cucumber seed treatment with the endophytic fungi Chaetomium Ch1001 had the highest potential against M. incognita infection. Fusarium oxysporium strain Fo162 synthesized several compounds that have a nematicidal effect on M. incognita (Hu et al., 2013). To date, the research on using endophytic fungi for managing phytonematodes is still rare. Hence, the present research aimed to evaluate the nematicidal activity of three endophytic fungi isolated from Taify rose against root-knot nematode, Meloidogyne javanica, in the laboratory.
MATERIALS AND METHODS
This investigation was carried out in the Department of Biology, College of Science., Taif University, Kingdom of Saudi Arabia.
Fungal filtrate preparation:
Three endophytic fungal strains, Aspergillus niger MK713445, A. niger MN513383, and Penicillium citrinum MN518391 isolated from leaves of Taify rose and previously identified molecularly were cultured on PDA medium. Agar discs from the 10 d-old PDA cultures of three fungus isolates were inoculated into 250 ml Erlenmeyer flasks containing 150 ml potato dextrose broth and shaken at 100 r/min at 28 °C for 10 d. The cultures were then filtered through Whatman filter paper (No. 1) and a 0.22 μm Micropore filter to separate the liquid from the mycelia. The filtrate was stored at 4°C until used.
Nematode culture:
Nematode inocula were obtained from a pure culture constructed from a single M. javanica egg-mass isolated from Taify rose roots that were previously identified according to its perineal pattern characteristics (Taylor and Sasser 1978) maintained and propagated on highly susceptible tomato plants c.v. Strain B in a greenhouse. Nematode eggs were extracted from infected tomato roots using 0.5% NaOCl solution and shaking for 2 min, while second-stage juveniles (J2s) were obtained by hatching method for seven days (Hussey and Barker, 1973). The inocula suspension was adjusted to 100 eggs or 50 J2s per ml.
Bioassay:
To test the in vitro toxicity of the fungal filtrates to eggs and J2s of M. javanica, 1 ml culture filtrates of each fungus isolate at three concentrations, i.e., 2, 4, and 8%; over 100 eggs or 50 J2s suspensions in 1 ml sterilized distilled water was poured into 24-well tissue culture plates. A 2.0 ml of potato dextrose broth and suspension of eggs or J2s served as control. Five replicates were used for both treatment and control, and the experiment was repeated once. The numbers of dead larva were recorded after 6, 12, and 24 h. J2s death was confirmed if their body appeared no realistic movement when touched with a fine needle. Abbott’s formula (Abbott 1925) was used to correct mortality rates (M): , where Mt= mortality percentage in treatment and Mc= mortality percentage in check. The rate of egg hatching inhibition in M. javanica was monitored under a light microscope at ten days of exposure. Hatch inhibition (HI) was calculated according to the formula: where C and T are the percentages of eggs hatched in the control and treatment, respectively. The characteristics of M. javanica J2s were examined under the stereomicroscope after 24 h exposure to the highest concentration of the three fungal filtrates or control (Unexposed treatment).
Analysis of fungal filtrates using gas chromatography-mass spectrometry (GC-MS):
Identification of the chemical constituents of filtrates of fungal isolates was analyzed by Gas Chromatography-Mass Spectrometer (GC-MS), Agilent Model 7890A-5975B [Column, DB 5 ms, Agilent form (30 m x 250 µm x 0.25 µm)]. The column was initially maintained at 40o C for 2 min, then raised to 50o C at a rate of 4o C/ min and held for 3 min, then raised to 150o C at a rate of 10o C/ min and retained for 3 min, then raised to 220o C at a rate of 10o C/ min and retained for 6 min, finally increased to 280o C at a rate of 15o C/ min and held for 10 min. The carrier gas used in this column was pure Helium (99.999%) for 10.9 min with a flow rate of 0.5 ml/min, then 1 ml/min per min to 1 ml/min for 30 min. Neither internal nor external chemical standards were used in this chromatographic analysis. The mass spectrum data per each chromatograph peak were interpreted using a computerized library-searching program (Willey 9 and NIST library) for the identification of the chemical constituents of fungal filtrates.
Statistical analysis:
Data were subjected to two-way ANOVA and Duncan’s multi-range test (p < 0.05) to compare the treatments using the COSTATE software package.
RESULTS
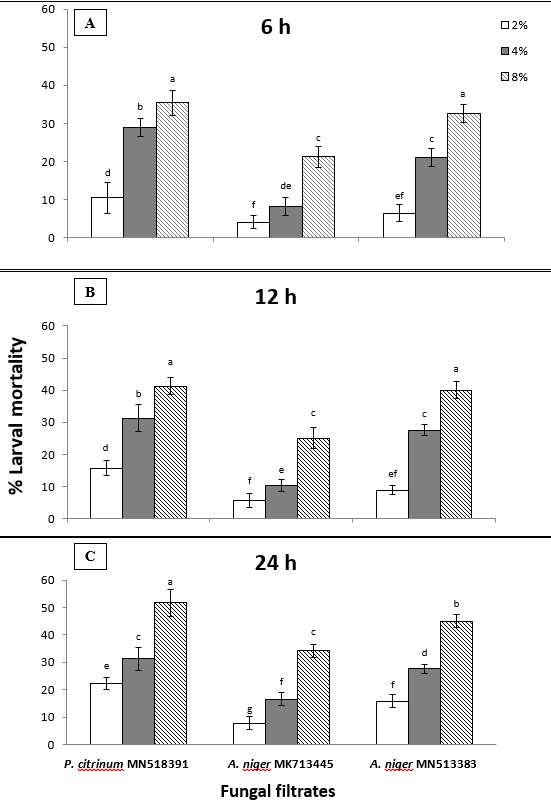
- Larval mortality:
The fungal filtrates of Aspergillus niger MK713445, A. niger MN513383 and Penicillium citrinum MN518391 at concentrations of 8, 4, and 2% in comparison with potato dextrose broth media on mortality percentage of newly hatched juveniles of M. javanica after 6, 12, and 24 h are shown in figure (1). Data revealed that larval mortality percentages increased with increased fungal filtrates concentrations and exposure periods tested. Exposition to culture filtrate of P. citrinum at 8% for 24 h significantly gave the highest reduction in the number of viable M. javanica J2s to be 51.67, followed by A. niger MN513383 at the same concentration and duration (45%), whereas J2s mortality reached 34.17% when exposed to culture filtrate of A. niger MK713445 (Fig. 1C). As illustrated in Fig. 1A&B, the number of viable J2s following six or 12-h exposure to 8% P. citrinum filtrate did not vary significantly from the number of viable J2s following exposition to A. niger MN513383 for the same times and concentration according to two way ANOVA, since it gave 35.42 and 32.5%, respectively after 6 h exposure; and 41.25 and 40%, respectively following 12 h exposure. Incubation of J2s for 12 or 24 h in the presence of P. citrinum resulted in remarkable larval mortality with mean reductions of 31.28% each at 4% and 15.79 or 22.27% at 2%, respectively. Exposition of J2s for 12 or 24 h to the middle concentration of A. niger MN513383 resulted in the same mortality percentage (27.57%). It was noticed that low concentration of both A. niger isolates did not act as good nematostatic since it reduced the number of viable J2s by 6.43, 8.91, and 15.79% or 4.02, 5.67, and 7.7% after 6, 12, and 24 h exposing to A. niger MN513383 or MK713445 isolates, respectively.
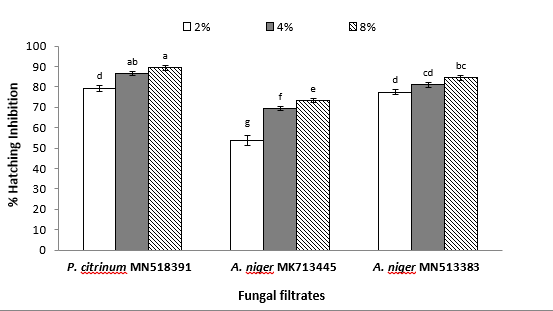
- Egg hatching:
The susceptibility of M. javanica eggs concerning hatching following exposure to fungal filtrates was demonstrated in figure (2). Likewise, higher concentrations of the three tested fungi resulted in significantly higher egg hatching inhibition percentages. Moreover, a similar trend concerning P. citrinum was recorded since it significantly ranked first in inhibiting egg hatchability that was 89.27 and 86.49% for concentrations of 8 and 4%, respectively. However, exposing M. javanica eggs to A. niger MN513383 filtrate reduced hatching by 84.41 and 81.05%, respectively, at the same concentrations. No significant difference in the number of hatched eggs was recorded between P. citrinum and A. niger MN513383 at 2%. The percentages of hatching inhibition of M. javanica eggs, which were exposed to A. niger MK713445 at 8, 4, and 2%, were considerably less than eggs under P. citrinum and A. niger MN513383 conditions (73.28, 69.35, and 53.8%, respectively).
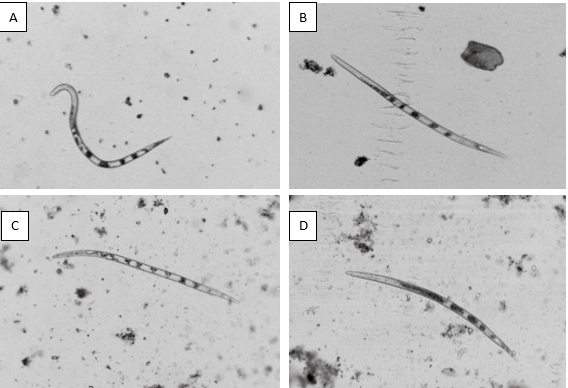
- Characterization of J2s:
Visual analysis of nematodes revealed that most J2s in control were mobile and retained the sigmoidal (∑-shape) characteristic typical for alive nematodes. Conversely, a significant reduction in nematode viability could be observed after exposition to P. citrinum culture filtrate, characterized in higher portions with straight body shapes (Fig 3B). Moreover, after exposing either to A. niger MK713445 or A. niger MN513383 (Fig 3C and 3D), the nematodes mostly exhibited the typical bent body (banana-shape), implying a reduction in viability.
- GC-MS analysis of active fungal filtrates:
This work subjected filtrates of the three target fungal isolates to GC-MS analysis. A total of 22 chemical compounds were found from the filtrates of the fungal isolates. The chemical composition of the filtrate varied from fungi to other, with the same number of compounds was found in all tested isolates (10 compounds); however, a few of them were predominant (Table 1). The results showed that the most bioactive compounds produced in major amounts by A. niger MN513383 were 6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline, hexadecanoic acid (Palmitic acid), and octadecane representing 64.895%, 7.437%, and 6.108%, respectively. The components of P. citrinum MN518391 also consisted of 6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline (65.334%), palmitic acid (7.973%), and pentadecane (6.370%) as major components. Similarly, 6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline (58.889%), acetic acid, piperidide (15.037%), and triacontane (6.081%) were abundantly present in the filtrate of A. niger MK713445. In fungal isolates, other compounds such as octadecane (1.875%), docosane (1.593%), phytane (1.397%), and squalane (1.088%) have been reported to be a common minor component. A small amount of 10-Methoxy-nb-alpha-methylcorynantheol was only detected in A. niger MN513383 (1.548%), whereas tetracosane (1.856%) was detected only in small amounts by P. citrinum MN518391. On the other hand, 1,1,2,2-Tetrachloroethane (1.654%) was estimated only in A. niger MK713445 (Table 1).
DISCUSSION
Screening entophytic fungi isolated from Taify rose leaves for the potential to be used as a bio-agent against M. javanica was examined in the laboratory. Fungi have been appeared to be the most important control agents for regulating nematode numbers in soil (Chen and Dickson, 2004). In the present investigation, M. javanica J2s mortality percentages significantly increased as the concentrations of fungal filtrates and tested exposure times increased compared to control treatment. Among all tested fungi, P. citrinum significantly reduced the number of viable J2s and egg hatching, indicating that nematicidal compounds were produced in the potato dextrose broth. This result was according to (Gotlieb et al., 2003), who recorded that incorporation of dry mycelium of P. chrysogenum into soil enhanced cucumber and tomato plant growth and reduced root galling caused by M. javanica. Several fungi regulate the nematode densities in soil by exhibiting a range of antagonistic activity, including the production of nematoxic compounds (Lopez-Llorca and Jansson, 2006). Many endophytes have secreted specialized metabolites and complex glycoproteins (Miller et al., 1998; Bashyal et al., 1999; Woropong et al., 2001; Castillo et al., 2002; Ezra et al., 2004), and some endophytic fungi emit volatile organic compounds (VOCs) (McAfee and Taylor, 1999; Morath et al., 2012; Stinson et al., 2003; Strobel et al., 2011; Mari et al., 2012) that may be biologically active, for instance, as a biofumigant for postharvest disease prevention (Suwannarach et al., 2013). Muscodor albus is an endophytic fungus that exhibits nematistatic and nematicidal properties against plant-parasitic nematodes (Riga et al., 2008; Grimme et al., 2007). VOCs produced by Fusarium oxysporum insulated from coffee plants rhizospheres were nematicidal to M. incognita (Freire et al., 2012). No significant difference was recorded between the high concentration of A. niger MK713445 isolate and median concentration of A. niger MN513383 isolate in reducing J2s viability after 6 and 12 h of exposure. Previous records reported that nematicidal toxins produced by the species of A. niger were effective against Meloidogyne while Penicillium produced toxins active against Aphelenchoides composticola (Cayrol et al., 1989; Grewal et al., 1989). Lower and median concentrations of A. niger MK713445 had no significant effect on J2s viability at all exposition durations. These results disagree with those recorded by (Jang et al., 2016), who mentioned that the culture filtrate of A. niger F22 was highly active against M. incognita with remarkable inhibition of egg hatching and J2s mortality. The result of the compound assay from the filtrates of fungal isolates using GC-MS shows several compounds contained in the filtrates. Based on the identification result using GC-MS, the characterized compounds belonged to alkane hydrocarbon, fatty acid, alkaloid, aliphatic hydrocarbon, aromatic hydrocarbon, heterocyclic amine, carboxylic acid, chlorinated hydrocarbon, and ketones groups. It is well known that several heterocyclic compounds containing nitrogen as quinazolinone derivatives exhibited a wide variety of biological activity. In the present study, high amounts of 6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline were detected in all tested fungal isolates. The highest percentage of this bioactive compound was recorded from P. citrinum MN518391, which had the best nematicidal properties in our study. These results agreed with Zheng et al. (2012) who mentioned that quinazoline alkaloid produced by the endophytic fungus Penicillium vinaceum, isolated from the corm of Crocus sativus, exhibited potential anticariogenic and antifungal activities. El-Gazzar et al. (2009) recorded that the quinazolinone derivatives have emerged as antimicrobial agents of immense interest because of their broad spectrum of in vitro and in vivo chemotherapeutic activities. Even though major components are usually responsible for the nematicidal activity of fungal filtrates, the effect of minor ones cannot be neglected. Results express the presence of minor bioactive compounds detected in fungal filtrates during GC–MS analysis. Out of which, fatty acids viz. hexadecanoic (palmitic) acid was identified in P. citrinum MN518391 and A. niger MN513383, dodecanoic acid in A. niger MN513383 and palmitinic acid in A. niger MK713445. Bardhan et al. (2019) stated that the predominant fatty acids detected from P. citrinum isolate PKB20 were linoleic acid (33.14%), oleic acid (30.09%), and hexadecanoic acid (20.25%). Likewise, hexadecenoic acid and hexadecanoic acid were the major compounds detected from hexane and ethyl acetate fractions of the rhizobacteria, Pseudomonas jessenii strain R62, and P. synxantha strain R81 and may be responsible for the nematicidal activity (Sharma et al. 2018). Our results were also confirmed with the record of Zhang et al. (2012), who reported that butyric, caprylic, capric, lauric, myristic, palmitic, and oleic acids showed the nematicidal action differently, among which capric acid exhibited a strong nematicidal effect and might be a powerful active substance for integrated M. incognita management. Elsewhere, Oliveira et al. (2009) signaled that palmitic acid is known to be very toxic to different nematode species. Triacontane and dotriacontane are the long-chain alkanes that were present as detectable content in the tested fungal isolates A. niger MK713445 and P. citrinum MN518391, respectively. Triacontane compound has recognized antibacterial and antifungal activities (Bordoloi et al., 2017). Small content of 1,1,2,2-Tetrachloroethane was present in the filtrate of A. niger MK713445. This compound is a chlorinated hydrocarbon and exhibited a pronounced nematicidal activity on intestinal nematode parasites. A single dose of 0.12 ml per kg (maximum of 5.0 ml) cured 80% of Necator americanus and 25% of Ancylostoma duodenale infections and may stimulate ascarids to migration (Marsden and Hoskins 1966). Another compound with minor content was 4-Oxopentanoic acid, p-tolylsulfonylhydrazone, ethyl ester recovered from A. niger isolate MK713445 and classified as a keto acid. Shemshura et al. (2016) reported that antagonism of the fungus A. candidus against M. incognita is primarily due to the production of two major compounds that have been identified as citric acid (Compound 1) and 1,2- dimethyl citrate (3-hydroxy-5-methoxy-3-(methoxycarbonyl)-5-oxopentanoic acid) (Compound 2). When compound 1 and a citric acid standard were tested at 50 mg mL−1 in water, hatchability of M. incognita eggs was decreased by more than 94%, and completely immobilized second-stage juveniles after 4–6 days exposure. Hawranik and Sorensen (2010) characterized 1,3-dimethyl citrate from A. niger and noted that it is also a constituent of numerous higher plants. The phenotypes of M. javanica J2s, either exposed or unexposed to fungal filtrates, were interesting findings that might be useful for analyzing the major mode of toxic action of these bio-control agents. The present result indicated that the nematodes exposed to potato dextrose broth (Control) was viable and retained sigmoid (∑-shape); while the nematodes treated with fungal filtrates mostly appeared paralyzed or dead and followed straight or bent shapes, similar to those killed by the pyrethroid that affects the central nervous system of the nematodes. This finding is according to those reported by Wiratno et al. (2009) and Nour El-deen and Issa (2016), who mentioned that the shapes of the dead nematodes differed characteristically, and groups of pesticides or bio-agents could clearly be distinguished based on this phenomenon. Our results represented examples of endophytic fungi with nematicidal properties and their modes of action, which introduced a reliable base for promising nematode bio-control agents. Further studies are necessary to isolate the endophytic fungi from other plant hosts and detect the secondary products that have nematicidal activity under greenhouse and field conditions.
| P. citrinum MN518391 A. niger MK713445 A. niger MN513383
|
| A |
| % Larval mortality |
| Sterile mycelium |
Fig. (1): Effect of endophytic fungi culture filtrates on M. javanica J2s mortality
| P. citrinum MN518391 A. niger MK713445 A. niger MN513383
|
Fig. (2): Effect of endophytic fungi culture filtrates on M. javanica egg hatching
| A |
| B |
| C |
| D |
Fig. (3): Characteristic shapes of M. javanica J2s following exposure to: A. Potato dextrose broth (Control)-sigmoid (∑-shape), B. P. citrinum MN518391-straight (I shape), C. and D. A. niger MK713445 and A. niger MN513383-bent (banana-shape).
Table 1. GC-MS analysis of the active components (in % Relative Content) in filtrates of fungal isolates
| Active compounds | A. niger MN513383 | P. citrinum MN518391 | A. niger MK713445 |
| Octadecane | 6.108 | 1.875 | — |
| 11-Butyldocosane | 4.333 | — | — |
| Heptacosane | 3.933 | — | — |
| Squalane | 1.088 | 4.921 | — |
| Dodecanoic acid | 4.704 | — | — |
| Hexadecanoic acid (Palmitic acid) | 7.437 | 7.973 | — |
| 10-Methoxy-nb-alpha-methylcorynantheol | 1.548 | — | — |
| 3-Methyl-2-butenoic acid,
2,7-dimethyloct-7-en-5-yn-4-yl ester |
2.702 | — | — |
| Docosane | 3.251 | — | 1.593 |
| 6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline | 64.895 | 65.334 | 58.889 |
| Boric acid, ethyl-, didecyl ester | — | 2.494 | — |
| Dotriacontane | — | 2.715 | — |
| Eicosane | — | 5.065 | 3.442 |
| Pentadecane | — | 6.370 | — |
| Phytane | — | 1.397 | 4.152 |
| Tetracosane | — | 1.856 | — |
| Triacontane | — | — | 6.081 |
| Acetic acid, piperidide | — | — | 15.037 |
| Palmitinic acid | — | — | 4.609 |
| 2-Methyl-,2-ethyl-2-[[(2-methyl-1-oxo-2-propenyl)oxy]methyl]-2-Propenoic acid | — | — | 2.462 |
| 1,1,2,2-Tetrachloroethane | — | — | 1.654 |
| 4-Oxopentanoic acid,
p-tolylsulfonylhydrazone, ethyl ester |
— | — | 2.081 |