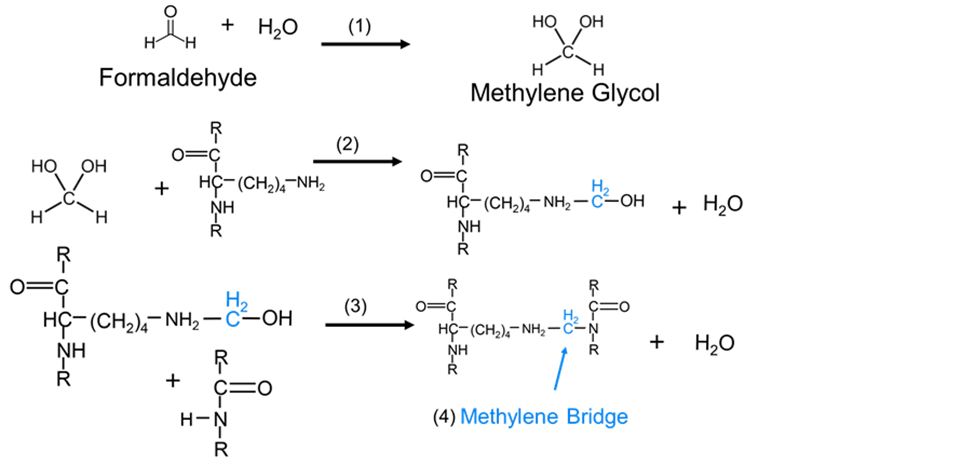
Methylene Bridge Process
The methylene bridge process is common in organic chemistry under the process called the methylation process. Methylation is the process through which a methyl group is added to a substance or a substitution of an atom or group by a methyl group. On the other hand, methylation is a form of alkylation process in which a methyl group replaces a hydrogen atom. Therefore, in organic chemistry methylene bridge, or methylene spacer or methanedily group is any part of a molecule that contains –CH2-. The methylene bridge process is that specific process through which molecules containing –CH2- are formed. The process occurs when a carbon atom is bound to two hydrogen atoms. The connection between a carbon atom and hydrogen atoms is through single bonds to other distinct atoms in the rest of a molecule.
The methylene bridge process occurs when there is a reaction of its active methylene group. This process is experienced when carbon atoms are bonded to hydrogen atoms in a repeating manner forming active methylene molecules. Therefore, carbon atoms and hydrogen atoms are the basic compounds that are used in the process. Methylene is a colorless gas that is soluble in water. It reacts with atmospheric hydrogen to form saturated hydrocarbon methane. Methylene undergoes rapid oxidation which results in the formation of water and carbon monoxide.
The product of the methylene bridge process is the formation of alkanes. Alkanes are organic compounds that have a single bond between the carbon and hydrogen atoms. Alkanes are termed as the simplest form of hydrocarbon family compounds that contain carbon and hydrogen with the carbon-carbon in a single bond. These alkanes are less reactive as compared to other chemical species. This is due to the carbon atoms in alkanes have obtained octet bonding through coverlet bond. Since their dormancy in reaction, they have little biological activity.
Reactions of Carbonium ions in the Phenol-Formaldehyde reaction gives rise to Methylene bridge process.
Examples of compounds which contain methylene bridges include: