Role of Polymers in Commercial Products
Product 1: Creams
Polymers working as Emulsion Stabilizers to produce cream
Emulsifiers help in keeping unlike substances like oil and water from separating. Most cosmetic products are mainly based on the emulsions-the small droplets of oil that is dispersed in water or the small droplets of water that have been dispersed in oil. Emulsifiers are thus added so as to change the surface tension between the oil and water thereby producing a homogenous product that has an even texture. Examples of these include polysorbates, laureth-4 and potassium cetyl sulfate.
The Hydrophobically-modified hydrophilic polymers also referred to as acrylates C 10-30 alkyl acrylate cross polymer was first introduced as being an emulsifier for the cosmetic products such as cream in the 1980s. Three has continued to be a growth in hydrophobically-modified polymeric emulsifiers that are based on poly (2-acrylamido-2-methylpropanesulfonate) (AMPS). Thus a combination of AMPS copolymer together with nonionic emulsifier leads to stable oil-in water emulsion lotions as well as creams which have sensory qualities and a non-tacky feel. The copolymer used is Genapol T-080. The inverse lattices of the copolymer of (2-acrylamido-2-methylpropanesulfonate and N, N-dimethyl acrylamide have been continuously used as patented as emulsion stabilizers as well as rheology modifiers.
The application of creams of the cosmetic emulsions leads to control of payout and also uniform application of the emulsion on the skin surface (Van et al., 2018). Inclusion of polysaccharides in the oil-water emulsion roll on products results into a lack of sticky feel. In addition to this, anionic polysaccharides are claimed to be selected from the aluminum starch octenylsuccinate, calcium starch octenylsuccinate and sodium starch glycolate. The copolymers of polyacrylamide and sodium acrylate are hydrophobically modified by a reaction with alkyl-amines. Copolymers that are produced by this particular route are essential as they enhance foaming properties of the shampoos. There are cationic polymers that easily form phase separated complexes together with anionic surfactants at a low surfactant concentration and these complexes are then solubilized at high concentration of the anionic surfactants. It is this particular phenomenon that forms the basis of the conditioning shampoos and then applied to the skin cleansing compositions which deposit beneficial materials on the skin after rinsing. This particular line of thinking has been made possible because of the introduction of cationic cassia derivatives.
Cassia gum is mainly a polygalactomannan isolated from the endosperm of seeds of cassia tora and Cassia obtusifolia. Cationic Cassia is a hydroxypropyltrimonium derivative of the Cassia gum. They are mainly used as coacervate-formers for the conditioning shampoos and also used as thickeners for both creams and lotions as well as suspension agents for the particulates in products like shower gels, cleansers of the skin and masks (Xue et al., 2019). Copolymers of diallylydimethlammonium chloride and acrylamide that have been advanced as coacervate depositing agents for skin. As an added advantage, they also allow low viscosity and or rheology to personal care compositions where they are included. Block copolymers that consist of polyionic blocks and neutral blocks are advanced as beneficial for purposes of deposition from the aqueous solution to the surface of the skin and hair.
The Rheology modifiers for the “Wet Skin”.
The moisturizing lotions applied during bathing are essential because they are applied to the wet skin and are expected to enhance moisturization. This is because the products can easily condense two processes to one- application of lotion and bathing, thus viewed as convenient and time saving products. There are also inventions that are directed towards the application of oils on the skin in the shower and also in the bath. The products are mainly effective if the beneficial agent is retained after drying with towel and after rinsing.
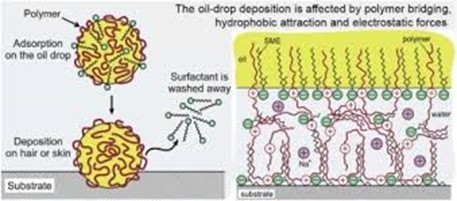
Diagram 1: The deposition of oil drop on solid surfaces in a mixed polymer-surfactant solutions in relation to hair and skin care applications
Graft copolymers
Gradient polymers that have low distribution of the molecular weight and composition and this has allowed the synthesis of gradient polymers. The gradient copolymers are those that have each end being rich in one or more of the monomer units and the polymer composition changes with the molecular chain from on monomer to the other. These types of polymers are essential to confer the conflicting properties in the same system and at the same time maintain stability. An example of the conflicting properties in the skin make up properties include matteness or gloss, resistance to the mechanical transfer, a good adhesion to the support and also removal by secreted sweat after it has been used. These particular conflicting properties can be greatly achieved by a blend of the different polymers however different polymers cannot mix at the molecular level and phase separation results.
EMT polymer
The EMT polymer is a multifunctional polymer that functions by swelling in the water and this is perfect for light, elegant cold-process lotions, serums and creams and it leaves the skin soft and conditioned.
Product 2: Shampoo
While in search of the very best personal care products, most consumers and buyers always reach out for the best product that combines a variety of properties. The combination consists of products that have a fair price, an appealing fragrance and a good feel. This knowledge is also applied when searching for the best shampoos in the market. However, did consumers really know where and how the shampoos come from? In this part of the blog, I discuss the effects and properties of shampoos and the manner in which they use polymerization to bring out the very best product. One of the best things about polymers is that they can be modified to perform different functions as well as serve different purposes.
The conditioning polymers such as the cationic polymers are examples of polymers best used for the hair. The polymers are mainly used as emulsion stabilizers as they help in the maintenance of the oil-in water formulation and this is beneficial for the hair products such as shampoos (Dias et al., 2016). Examples of these polymers include carbomer and Carboxyl methyl. The major parameter correlating the thickness and flow of shampoo is the viscosity. Viscosity is the ability of a liquid to refuse to flow. Viscosity affects the perception of the user and the efficiency of cleaning. Viscosity is of great importance when finding out the best choice of shampoo.
In today’s industry, most consumers also look out for the shampoos that can serve more than one purpose. Apart from cleaning the hair, they also serve the purpose of smoothing it as well as improving its compatibility. Cationic polymers are most commonly used to serve this purposes. With their high molecular weight and being effective as anionic surfactants, they are used due to their powerful cleansing ability (Dais et al., 2016). The polymer residue that is used for conditioning of the hair doesn’t form flat layers on the hair, however, it absorbs the cationic moieties making the uncharged part of the polymer to form a loop and this is then oriented away from the hair and this is mainly essential as it helps to reduce friction that may occur between the hair. Apart from this, silicones are also used as essential ingredients for conditioning. The polymers deposit towards the surface of the hair and this allows a reduction in friction thereby providing an emollient effect and also helping in the reduction of the static charge between the strands of the hair (Nicholson, 2016).
The ability to designate shampoos has over the years continued to change from the 2 in 1 to 3 in 1 multifunction. This is because for most of the consumers its demands live up to their promises when it is a 3 in 1 as seen in the figure 2. The modern shampoos have a great number of ingredients such as the pro-vitamins, vitamins and also the silicones. The polymers used are normally branched and unbranched and this allows thickening effect due to the formation of cluster. The 2 in 1 functionality is achieved by mixing cationic polymers in the anionic shampoo and suspension of the silicone in the anionic shampoo.
Figure 2: Example of a 3 in 1 shampoo
The conditioners are held in suspension by the polymers that are mainly used to bind to the surfactant molecules allowing conditioning molecules to come together (Li & O’Connor, 2016).
When an individual rinses their hair, the surfactant separates from the conditioner and the polymer. This happens because both the conditioner and the polymer are insoluble in water. Regardless of the type of formulation, in most cases consumers always have different opinions for the same product. This is because most regard viscosity as they put different energy while squeezing through the shampoo bottle. Thus, manufacturers are in position to modify the behavior of flow in a bid to attract different types of customers. This is mainly achieved because of the use of water soluble polymers which are used as modifiers. With the environmental concerns. Researchers also look out to those shampoos that are environment friendly. Thus, with evolving research, scientists have been able to come up with synthetic fibers which are found naturally in seafood shells derived from chitin polymer. These types of polymers are normally found occurring naturally and used to make shampoos, laundry agents and other cleansing agents.
Product 3: Soaps
Soap-an excellent surfactant
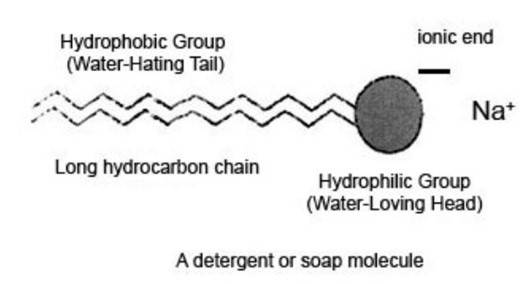
Soaps were the earliest surfactants and are obtained from fats referred to as glycerides since they are esters formed by the trihydric alcohol, propanone-1, 2, 3-triol (glycerol) that has long chain carboxylic acids. Surfactants are mainly used to break down the interface between oils or dirt with water. They are also able to change the manner in which water behaves. When it is added to a surface, the surface tension is reduced. Water then spreads out and wets the surface such as clothes and dishes. They function by breaking down the interface that exists between the oils and water thereby they hold the oils and dirt in the suspension and then bring about their removal. This property is made possible because of the presence of a hydrophilic group such as the acidic anion and the hydrophobic group such as the alkyl group that hates water, Molecules of water congregate near the former (Schumann& Siekmann 2005).The process of the formation of soaps is referred to as saponification. Soaps contain a long hydrocarbon chain which is oil soluble and a short ionic end of sodium ion that is water loving.
Diagram 3: Structure of a soap molecule
Saponification and the Emulsifying property of soap
Being an excellent cleanser, soap is able to act as an emulsifying agent. An emulsifier is able to disperse one liquid into another immiscible liquid. This therefore means that soap easily suspends in a manner that allows it to be removed. Its organic part is negatively charged polar molecule and its hydrophilic end allows the interaction in the water molecules through ion-dipole interactions and hydrogen bonding. The long hydrophobic end does not interact with the water molecules. The hydrocarbon chains are attracted to each other because of the dispersion forces as well as cluster together and this allows the formation of micelles. Since dirt is a non-polar and insoluble in water it allows the micelles to break up the non-polar molecules.
High temperatures and mechanical agitation allow the dispersion of the soil or dirt into the wash water. In the step, soil and dirt is then dispersed in the foam formed by the mechanical action of the hands. To be able to prevent the dirt from being deposited to the surface that has been cleaned, it is suspended in a protective colloid with the help of special additives. Most of the soiled surfaces allow the dirt to be bound to the surface because of a thin film of oil or grease. The cleaning of these kinds of surfaces mainly requires displacement of the film by detergent solution as it is then washed away by the rinse waters (Cao et al., 2019). The oil and dirt film then breaks up and then separates into individual droplets by the help of detergent solution.
During the process, the surfactant is added to the water and the water fearing end stays warm from the water. This is achieved by organizing into the shape of a sphere and the water loving end is then found positioned on the outside while the water fearing ends is protected on the inside. The spherical shape of the surfactant is referred to as a micelle. It is an important part because it traps the dirt such as soil. Since the inside is hydrophobic, then the dirt such as soil also automatically becomes hydrophobic. The attraction of the soil in the inside of the surfactant micelle allows the loosening of the soil from the surface (Bartels et al. 2005). Once the soil then lifts off the surface, it now becomes suspended in the water inside the micelle. The suspension is thus referred to as emulsification of one of the liquids into the other. The soil or any other dirt no longer settles back to the surface but remains inside the micelle. The soil or any other dirt is then trapped inside the micelle and the micelle is then suspended in the water making it easy to wash the dirt away.
The glycerides that are used for the manufacture of the soap contain both the saturated and unsaturated carboxylic acids and these have an even number of carbon atoms for example the stearic acid. Soaps normally form insoluble calcium and magnesium salts and this is a great disadvantage as compared to the synthetic surfactants (Hoover, 2016). This is because of the formation of a scum present the dirt and this ends up wasting the soap. This can be however avoided by the use of synthetic surfactant. In the anionic surfactants, the carboxylate group in the soap is normally replaced by a sulfonate in the hydrophilic component.
The calcium and magnesium are more soluble in the water as compared to the calcium and magnesium salts present in the carboxylic acids. The invention allows the novel water soluble polymers and dispersants as well as detergent compositions to be formed (Thorsten, 2015). The water soluble polymers are mainly used as detergent compositions and serve as builders and lime soap dispersants because they sequester the hard water. The water soluble polymers are essential in liquid detergent compositions and commercial home liquid dishwashing products.