Substitution Reactions of Alcohols and Reaction Rate Studies of an SN1 Reaction
Synthesis of 1-bromobutane from 1-butanol
Objectives/ Purpose of the Experiment
The objective of this study was to synthesize 1-bromobutane from 1-butanol. The experiment also aimed at finding out the percentage yield of 1-bromobutane compared to the theoretical value. Lastly, the experiment was conducted to observe the BP of 1-bromobutane and compare it with literature.
Introduction
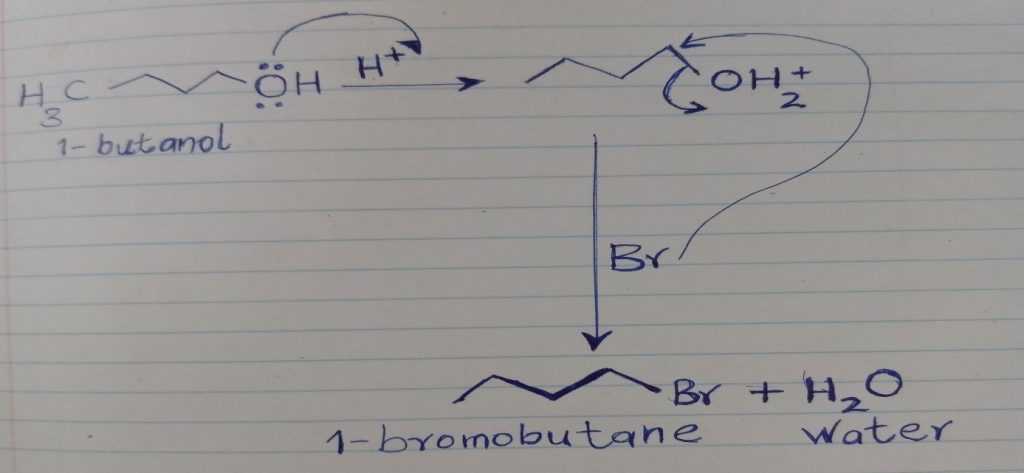
The experiment involved a nucleophilic substitution reaction. The reaction in this category was a second-order nucleophilic substitution reaction. In this reaction, three conditions were necessary; nucleophiles, electrophiles, and the presence of a free radical. The reaction’s progression through substitution required the nucleophile to be nearest such that the electrophile freely accessible, like in the 1o -CH3 (methyl) group. The leaving group also needed to be within an appropriate range. In this criterion, water acts as a leaving group in the acidic medium. The bromide ion’s presence fulfilled the need for a nucleophile, and the electrophile was presented as the 1o alkyl group. In this manner, the -OH group was used as an unfavorable leaving group. The reaction in the experiment was synthetic since a new product was formed.
Synthetic Equation
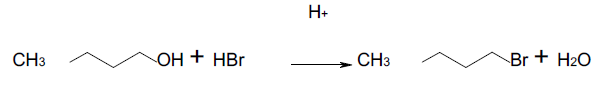
1-butanol was used as the main reagent in the experiment. 1- butanol is a primary alcohol. Therefore, the alcohol underwent the SN2 reaction with the acid (HBr solution), and the product was 1-bromobutane. Sulfuric acid was used as a catalyst but in a small quantity because it assisted to protonate the -OH functional group to form the leaving group (water). The figure below represents the synthetic equation for the reaction that occurred.
Figure 1. The synthetic equation for the synthesis of 1-bromobutane from 1-butanol
Note that the structures of the reagents and the products are also indicated. Concentrated sulfuric acid causes burns on the skin and needs to be handled with care. HBr solution irritates the skin, nose, and eyes; it required care while working with it.
In this reaction, the leaving groups are Br–, -OH, and Cl–, while the alkyl group is the butyl radicals formed. Phenolphthalein indicator was used in the experiment. The rate at which the acid was generated (turning the indicator colorless) represented the reaction rate.
Figure 2: General equation of the reaction
The synthesis occurred through a complex procedure that had various steps. After a stoichiometric mixture of the reactants, a reflux process was carried out, followed by separation using simple distillation. Some of the by-products of the reaction, such as sulfuric acid, were eliminated by washing with distilled water. Water was formed in the reaction as the leaving group, while Br- obtained from HBr was the nucleophile involved in the nucleophilic substitution reaction to form the target product (1-bromobutane)[1].
Table 1. Structures of reagents and products in the reaction
| Compound | Chemical Formula | Chemical Structure |
| 1-butanol (reagent)
A colorless liquid. 1-butanol is flammable and causes fires when near an open flame. The compound is corrosive and causes and irritation of the eyes[2]. |
C4H10O | |
| Hydrogen bromide (reagent)
Hydrogen bromide solution forms HBr fumes that are colorless and have a pungent smell. HBr solution is corrosive and irritates the eyes, skin, as well as the respiratory system.[3] |
HBr | |
| Concentrated sulfuric acid (catalyst) and (Solvent).
Concentrated sulfuric acid is an oily, colorless, water soluble inorganic liquid. Concentrated sulfuric acid readily chars organic compounds such as wood. The acid is highly corrosive and causes burns and irritation to the skin. However, the acid doesn’t burn (is not flammable).[4] |
H2SO4 | |
| Water (product) | H2O | |
| 1-bromobutane (product). 1-bromobutane is a colorless organic liquid with a higher density than water. The compound forms fumes that are denser than air. The compound is flammable and catches fire easily when close to open flames. The compound causes serious skin, eyes, and respiratory system irritation.[5] | C4H9Br |
Methodology
Materials and Reagents
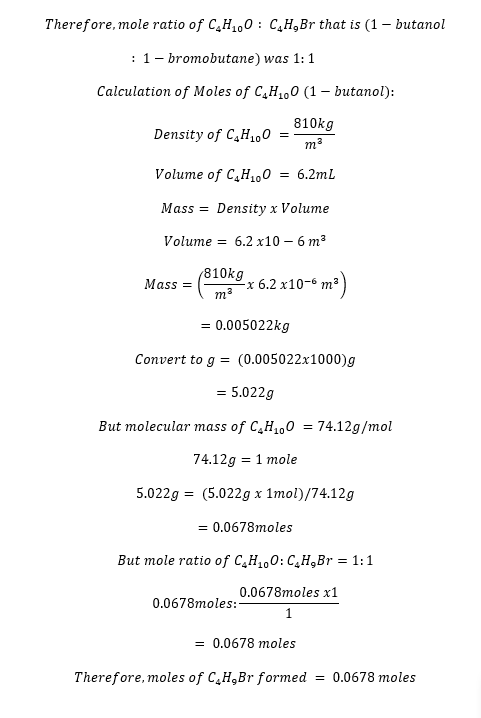
6.2mL 1-butanol (C4H10O)
10mL of 48% HBr solution
4mL of concentrated sulfuric acid
100mL distilled water
5mL of 5% Sodium bicarbonate
Apparatus
25mL Round bottomed flask
1000 mL Round bottomed flask
Pipette
Weigh balance
Stop watch
Thermometer
Reflux apparatus and gas trap
Erlenmeyer flask
Distillation setup
Ice bath
Boiling chips
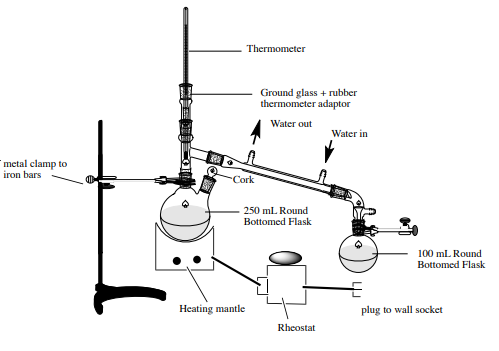
Figure 3. Set-up for the experiment
Procedure
A 100mL round-bottomed flask was obtained, and then 6.2mL of 1-butanol, 10mL of 48% HBr solution, and two boiling chips were added. A graduated cylinder was washed and used to measure 4mL of concentrated H2SO4. The concentrated H2SO4 measured was added slowly to the mixture. The reflux apparatus was set up with its gas removal (vacuum adapter) apparatus with no stopper while the hose was connected to the aspirator. The flask contents were heated at reflux for 45 min. While heating was continuing, a study of the leaving groups, solvent polarity, and alkyl groups was conducted. The mixture was allowed to cool down then 10mL of deionized water was added through the condenser at a slow rate. Two new boiling chips were added, and the apparatus was converted into a simple distillation. Distillation continues until the temperature of the distillate was 100oC (close to 10mL). The distillate was collected into a 25mL round-bottomed flask secured in an ice-bath. A transparent pipette was used to remove the aqueous portion of the distillate. The organic layer was washed in 5mL distilled water and then in 5mL of 5% sodium bicarbonate solution. The organic layer was washed again in 5mL distilled water. The organic layer was transferred into an Erlenmeyer flask. The 1-bromobutane formed was dried over anhydrous sodium sulfate. The product was pipetted from the drying agent (Na2SO4) and then weighed.
After the mixture was heated for 45 min in gentle reflux, two layers were observed. One layer at the bottom had a yellowish color contained mainly the product (1-bromobutane). Since 1-bromobutane was not soluble in concentrated sulfuric acid, it settled at the bottom of the round-bottomed flask. After heating was maintained for 45 minutes, it was minimized by putting the heating mantle further down from the flask holding the reactants. The funnel was immediately removed so that sucking up action was not started by the water from the bath into the reactant’s flask. The flask’s content was allowed to cool, and then distillation was carried out to separate the product from the impurities. An IR spectrum analysis of the product was conducted. Finally, the % yield of the product from the reaction was calculated.
Calculations
The balanced chemical equation for the reaction is as follows;
C4H10O (aq) + HBr (aq) à C4H9Br (aq) + H2O(l)
Summary of Results
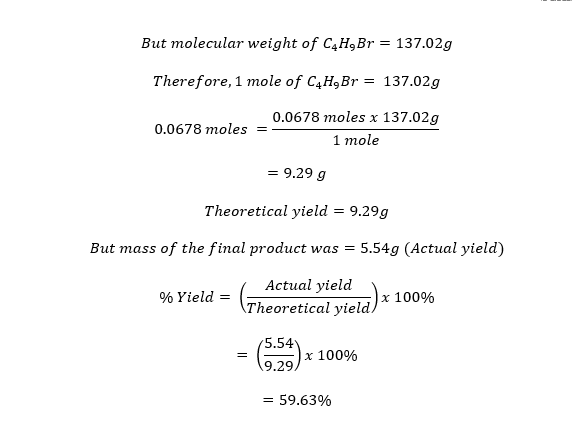
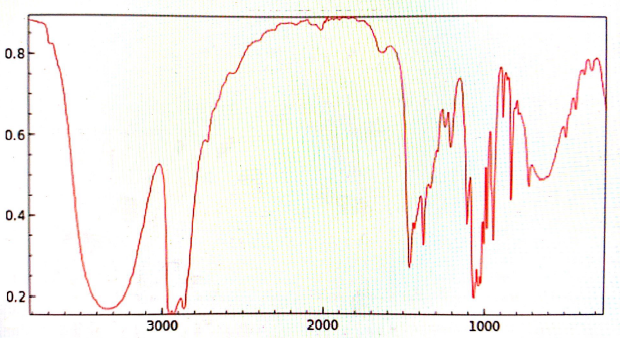
The final product was bromobutane and weighed 5.54g. An IR spectrum analysis of the product was as follows;
Figure 2. 1ST IR spectrum analysis
Figure 3. 2nd IR spectrum analysis
There were major peaks formed at 3000 cm-1, 1450 cm-1, and 1375 cm-1. The peaks were clearer in the 2nd analysis than in the 1st because more product than reagent was present in the second IR spectrum analysis. Therefore, the transmission spectrum analysis in the second instance indicated a purer product than in the first.
Discussion
When a mixture of 1-butanol, HBr, and H2SO4 was heated, HBr gas was produced. Concentrated sulfuric acid offered an acidic medium that allowed the protonation of the -OH group. Sulfuric acid acted as a dehydrating agent, combined with the water molecules formed from the substitution reaction. Sulfuric acid was also used to remove the organic by-product of the reaction, such as 1-butene, dibutyl ether, and the excess reagent (1-butanol). [6]
The experiment generated a substantial amount of the product. The purification of the product was achieved via distillation. Simple distillation was applicable as a separation method because bromobutane (product) and 1-butanol (reagent) have close but different boiling points. Since bromobutane had a lower boiling point, it was distilled first. The application of the distillation process as a method of purification was possible due to the difference but closeness in the boiling points between the product and the mixture of the by-products and the reagent. The purity of the product would be improved further by using an appropriate drying agent such as anhydrous calcium chloride. 6
A transmission IR spectrum analysis indicated a peak at 3000 cm-1. Therefore, the peak indicated the existence of sp3 hybridization, such as in alkanes. There was also a strong peak close to 1375 cm-1. In this case, the peak was caused by the methyl (-CH3) at the end of the bromobutane molecule. Lastly, there were two peaks close to 1450 cm-1 that were due to the (-CH2) group.6
The gas trap in this experiment was used to ensure that HBr fumes do not disappear to the environment where it could be inhaled and damage the lungs. Another preauction take was handling of concentrated sulfuric acid that can burn the skin and cause irritation. The reflux was applied to heat the product and avoid the escaping/loss of the fumes. The fumes were formed as condensation products and collected as liquids.
After the commencing of the reflux, the solubilities of the products in water are altered. For instance, the density of water and the product (1-bromobutane) are different; therefore, the denser is denser than water formed at the bottom. Therefore, the top layer contained water deprotonated by concentrated sulfuric acid. The bottom layer contained the 1-bromobutane.
The difference in BP between the reagent (1-butanol) and the product (1-bromobutane) allowed for the application of distillation as a method of purification/separation. The distillation was done at 100oC since bromobutane’s boiling point is 101.3 °C and formed vapor first. Therefore, it was easier to collect and condense the product’s fumes into a liquid before the fumes of the other organic part (mainly 1-butanol) formed.[7]
Concentrated H2SO4 acted as a strong acid. It was responsible for protonating the -OH (alcohol) group, converting it to a leaving group. Again, H2SO4 was used as a solvent in the experiment. It was used as the appropriate aprotic solvent that doesn’t form a hydrogen bond with a nucleophile. Water was used as a cleaning agent for the poler by-products. Since water didn’t dissolve the product (1-bromobutane), it was used as an appropriate solvent for cleaning the product. Sodium bicarbonate was used as a neutralizing agent for the acidic by-products. Sulfuric acid was also used in assisting the water molecules in shifting the equilibrium to the right (in favor of the formation of 1-bromobutane).[8]
The mass of the product obtained was significantly lower than the theoretical value. Therefore, much of the product must have been lost due to errors in the experiment. For instance, some 1-butanol may not have reacted but ended up being washed away as a solute dissolved in sulfuric acid. Such an error would be avoided by allowing time for the reaction to be complete.
The purity of the final product was also susceptible to experimental errors. The purity of the product may be improved by enhancement of the cleaning and drying process. Better drying agents such as anhydrous calcium chloride may be applied to improve water moisture elimination in the final product.
As noted earlier, the product (1-bromobutane) forms fume easily. Therefore, due to its high volatility, some of the products could have escaped to the atmosphere leading to having a lower experimental yield than the theoretical yield. Such an experimental error could be corrected using a ground grass stopper to close the round-bottomed flask for collecting the distillate. It should also be noted that some of the products could have also frozen and solidified around the stopper leading to recording a lower mass of the product. Using a stop cock grease would correct the experimental error associated with the product’s freezing around the glass stopper. The wastes were disposed of appropriately to prevent reactions that would affect the environment, such as between concentrated sulfuric acid and the working benches.8
Conclusion
The calculated % yield was 59.63%, which signified that 5.54g of 1-bromobutane was formed from the experiment. The experiment met its objectives since 1-bromobutane was produced from 1-butanol. The nucleophilic substitution reaction was involved in the formation of 1-bromobutane from primary alcohol. Caution was exercised throughout the experiment due to the reagents’ hazard and safety concerns and the product. For instance, open flames were avoided since both 1-bromobutane and 1-butanol are highly flammable. Skin contact and direct inhalation of HBr, H2SO4, and 1-bromobutane were avoided because they were corrosive and irritants. Appropriate disposal of the wastes into the sink was emphasized to prevent the reaction with the environment.
Here are some the chemistry writing services we provide:
– Chemistry assignment writing services
– Chemistry Essay Writing Service
– Chemistry Dissertation Writing Service