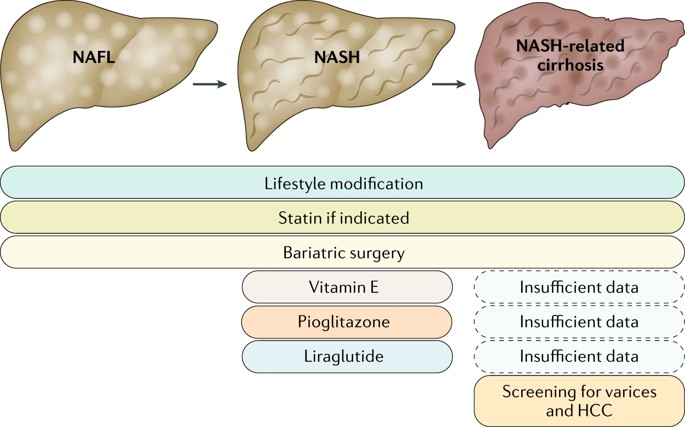
Treatment guideline for NASH
The current treatment protocol for NASH is established by the American Association for the Study of Liver Disease1. The first aspect of this guideline is to choose whom to treat in the spectrum of this disease, every patient does not require pharmacological treatment; although every patient needs general medical intervention. Only patients with biopsy-proven NASH and fibrosis require pharmacological treatment.
- Lifestyle intervention: consists of diet, exercise, and weight loss that have been advocated to treat Nonalcoholic fatty liver disease (Umbrella term to englobe NAFL and NASH). Many studies state that weight loss is a key component to improve the histopathologic feature of this disease. Diet modification is based on a calorie-restricted diet of 30% of calorie restrictions; also is suggested the Mediterranean diet as a good diet as an example to begin with.
Guidance statement: hypocaloric diet (daily reduction by 500-1000 kcal) and moderate-intensity exercise, weight loss of at least 3 to 5% of body weight, exercise alone in adults with NAFLD may prevent or reduce hepatic steatosis.
- Insulin Sensitizer:
- Metformin: many studies have proven improvement in aminotransferase values and insulin resistance. Although, several studies reported no improvement in liver histology.
Guidance statement: Metformin is not recommended for treating NASH in adult patients
- Thiazolidinediones: this drug is a ligand for the nuclear transcription factor PPAR-γ with broad effects on glucose and lipid metabolism, as well as on vascular biology and inflammation. Many randomized control trials have proven their positive effect on liver histology and metabolic functions. Doses used in the studies: Pioglitazone 30-45mg/day
Guidance statement: Pioglitazone improves liver histology in patients with and without T2DM with biopsy-proven NASH. Therefore, it may be used to treat these patients. Risks and benefits should be discussed with each patient before starting therapy. Until further data support its safety and efficacy, pioglitazone should not be used to treat NAFLD without biopsy-proven NASH.
- Glucagon-Like Peptide-1 Analogues: it’s a new drug and currently has been undergoing many studies to prove its efficacy. These results are promising but scientific community need more evidence to rely on.
Guidance statement: it is premature to consider glucagon-like peptide-1 agonists to specifically treat liver disease in patients with NAFLD or NASH.
- Vitamin E: oxidative stress is considered a key mechanism of hepatocellular injury and disease progression in subjects with NASH. Vitamin E is an antioxidant and has been investigated as a treatment for NASH.
Guidance statement: administered at a daily dose of 800 IU/day improves liver histology in nondiabetic adults with biopsy-proven NASH and therefore may be considered for this patient population. Until further data supporting its effectiveness become available, vitamin E is not recommended to treat NASH in diabetic patients, NAFLD without liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
- Bariatric Surgery: sustained weight loss is difficult to achieve and harder yet to sustain in the patient. Bariatric surgery improves or eliminates comorbid disease in most patients and improves long-term survival and death from cardiovascular disease (CVD) and malignancy, the 2 most common causes of death in NAFLD/NASH.
Guidance statement: Foregut bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. It is premature to consider foregut bariatric surgery as an established option to specifically treat NASH. The type, safety, and efficacy of foregut bariatric surgery in otherwise eligible obese individuals with established cirrhosis due to NAFLD are not established. In otherwise eligible patients with compensated NASH or cryptogenic cirrhosis, foregut bariatric surgery may be considered on a case by case basis by an experienced bariatric surgery program.
- Other therapeutic agents: newer studies are investigating the efficacy of some new or already known components (Ursodeoxycholic Acid, Omega-3 Fatty Acids). Current studies have shown promising results, but more research is needed with larger numbers of patients.
Guidance statement: Ursodeoxycholic acid is not recommended for the treatment of NAFLD or NASH. Omega-3 fatty acids should not be used as a specific treatment of NAFLD or NASH, but they may be considered to treat hypertriglyceridemia in patients with NAFLD
- Alcohol use in patients with NASH
Guidance statement: Patients with NAFLD should not consume heavy amounts of alcohol. There are insufficient data to make recommendations with regards to nonheavy consumption of alcohol by individuals with NAFLD.
- Management of Cardiovascular Disease and Dyslipidemia:
Guidance statement: Patients with NAFLD are at high risk for cardiovascular morbidity and mortality. Thus, aggressive modification of CVD risk factors should be considered in all patients with NAFLD. Patients with NAFLD or NASH are not at higher risk for serious liver injury from statins. Thus, statins can be used to treat dyslipidemia in patients with NAFLD and NASH
References:(1) The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis (Hoboken). 2018;11(4):81. Published 2018 Apr 20. DOI:10.1002/cld.722.
Epidemiology of NASH
The global population with NASH lay between 3% to 5%; in the United States of America (USA) the numbers are similar, an estimate of 2.6% to 5% of the population has NASH.
Currently, it doesn’t exist much data about the incidence of NASH, only a few countries had made epidemiological studies about this aspect. England reports an incidence of 29 per 100.000 persons, Israel an incidence of 28 per 1.000 persons, and a pooled regional study from Asia reports an incidence of 28 per 1.000 persons. There may be a high chance that these studies underestimate real numbers because of the methodology of diagnosing NASH, incidence indeed could be higher. Nevertheless, there is an interesting study5 that uses statistical methods for model prediction of NAFLD/NASH that predicts 3.62 million new cases annually.
The most common comorbidities in NASH patient are obesity, diabetes, and hypertension (cardiovascular); respectively the percentage of patients with NASH with this comorbidities are 31-95%, 46%, and 2-30%. The overall mortality rate in NASH was 25.56 per 1000 patients, with the liver-specific mortality rate reported as 11.77 per 1000 patients.
NASH and Treatment, controversies.
Article1: Ratzio reports the necessity of a line of specific drugs, and establishes the problem of who to treat with drugs in the context of these patients with NASH. NASH is now an established indication for therapy; irrespective of the control of associated metabolic comorbidities. Patients with histologically confirmed steatohepatitis and fibrosis are candidates for pharmacotherapy, in association with diet and lifestyle modifications. Current treatment options are limited, new, and innovative pharmacological agents are being developed. Obeticholic acid and elafibranor are the most advanced drugs that induced the resolution of NASH and for obeticholic acid, an overall improvement in fibrosis. Many other drugs such as chemokine blockers, inhibitors of lipogenesis, and antifibrotics are in various stages of development. However, all these mentioned drugs they lack big proportional studies that sustain the evidence of using them in the daily clinical practice.
Article2: Caldwell revised articles of the evidence of several older forms of therapy that have been fairly extensively studied over the years: Polyunsaturated Fatty Acid (PUFA) supplements, vitamin E, insulin-sensitizing agents with a focus on pioglitazone. For omega 3 supplements they appear to reduce liver overall fat within a 1-2 year time frame but neither reduce nor exacerbate steatohepatitis. the data on vitamin E suggest that high dose vitamin E (800 IU/day) appears to have some benefit in mild NASH among pediatric patients but only a limited effect in adults. Concerns regarding safety with the long-term use of the agent and its limited efficacy have dampened enthusiasm although it is notable that no evidence vitamin E related adverse events were reported during two years of therapy in these trials. The only insulin-sensitizing agent that had shown positive evidence of histology improvement is Liraglutide, a parenterally administered analog of glucagon-like peptide-1 (an incretin) compared to a placebo-treated group after approximately one year of therapy in the ‘LEAN’ trial. 9 of 23 (39%) treated patients versus 2 of 22 (9%) had histological resolution of NASH. Gastrointestinal side-effects were common in both groups but more so in the treated group. Further study seems warranted with longer follow-up and larger study groups.
Article3: recent guidelines don’t recommend any specific type of bariatric surgery. In this article, Jirapinyo P support the Gastric Bypass in Y-de-Roux as the gold standard surgery to treat patient with NASH. There is emerging evidence that bariatric surgery, in particular RYGB, is effective at treating NASH with improvement in histological features in the short term. Current guidelines suggest that it is premature for bariatric surgery to be considered an established treatment specifically for NASH alone, without concomitant obesity. Randomized controlled trials and long-term studies are underway to better clarify the role of these procedures specifically for NASH therapy.
Peachy Essay and its solid medical science writing help team consist of several medical doctors provides a wide range of academic writing services including:
– Medical science assignment help
– Medical science essay help
– Medical science dissertation help






