A LITERATURE REVIEW
Table 2: PICO Components and Clinical Question (Adapted from Purssell & McCrae 2020)
| Population (P) | Intervention (I) | Comparison (C) | Outcome (O) |
| Patients with chronic renal disease stages 2-4 | Low protein diet | Non restricted diet | Delay in disease progression |
| Clinical question: Does a low protein diet help slow down the disease progression in patients with chronic renal disease in stages 2-4? | |||
Table 2: Keywords and Boolean Operators
| Keyword
Population |
Keyword Intervention | Keyword
Comparison |
Keyword Outcome | |||
| Chronic kidney disease
|
Low protein diet
|
Non restricted diet | Delayed disease progression | |||
| OR | OR | OR | OR | |||
| Chronic renal disease
|
Protein restricted diet
(MeSH) |
Normal diet | Disease management | |||
| OR | OR | OR | OR | |||
| Chronic renal insufficiency
(MeSH) |
Protein limited diet
|
High protein diet | Slowed progression | |||
| OR | OR | |||||
| CKD
(MeSH) |
Dietary proteins
(MeSH)
|
|||||
| OR | OR | |||||
| Kidney disease*
(MeSH) |
Reduced protein | |||||
| OR | ||||||
| CKD stage* 2
|
||||||
| OR | ||||||
| Chronic kidney disease stage* 2-4
|
||||||
| OR | ||||||
| Nephritis ) | ||||||
| OR | ||||||
| Glomerulonephritis ) | ||||||
| OR | ||||||
| Human*
(MeSH |
||||||
| OR
|
||||||
| Adult*
(MeSH |
Table 3 Inclusion and Exclusion (Adapted from Aveyard 2012)
| Inclusion criteria | Exclusion criteria | Rationale |
| Abstract Available | Abstract Unavailable | To ascertain if selected articles are relevant to research question. |
| English Language | Non-English Language | To retrieve relevant articles written in the English language. |
| Publication 2010-2021 | Publication before 2010 | To retrieve information in the relevant timeframe of the last ten years. |
| Adult 19+ | Children <19 | To retrieve relevant articles that look at adults with CKD |
| Human | Animals | To retrieve articles that are relevant to the human species. |
| Full text | Restricted text | To retrieve articles that are fully available to read. |
Abstract
Chronic renal failure continues to pose significant health problems for many individuals and healthcare systems across the world. Several interventions, including pharmacological and non-pharmacological, have been developed to manage the condition. Low-protein diet (LPD) is one of the evidence-based non-pharmacological intervention that has been addressed in the literature as effective at delaying the progress of renal decline and subsequently chronic kidney disease. However, many clinicians still appear sceptical about the effectiveness of this intervention. The current study, therefore reviewed existing literature to establish the effectiveness of LPD on delaying the progression of CKD, especially among those with stages 2-4 CKD. The systematic review yielded 12 articles that were selected after being subjected to rigorous exclusion/inclusion criteria. Findings from all the articles reviewed showed that LPD interventions are effective in delaying the progression of CKD. The findings also showed that LPD is a safe intervention as long as the clinicians involve a multi-stakeholder approach involving nutritionists. In conclusion, it is recommended that clinicians should be sensitised about the effectiveness of LPD in helping patients with CKD, including how to best prescribe the intervention to achieve optimum healthcare outcomes.
Keywords: Chronic renal failure, chronic kidney disease, CKD patients, low-protein diet, CKD progression, delay progression
Introduction
In health, the kidneys are responsible for the excretion waste products, toxic substances, and drugs through the urine (Dharmaratne, 2019). Both acute renal failure (ARF) and chronic renal failure (CRF) are important public health issues because they can progressively lead to adverse healthcare outcomes including increased healthcare costs (Watanabe, 2017). Watanabe (2017) further noted that globally, the prevalence of CRF, also known as chronic kidney disease (CKD) is between 8% and 16%. The traditional causes of CKD include diabetes, hypertension, and other cardiovascular diseases. CKD condition is also associated with several inherent lifestyle-related healthcare changes including diet modification, exercise, fluid allowance etc. CKD complications include metabolic acidosis, heart diseases, fluid build-ups, and bone diseases (Schrauben & Berns, 2020). Several interventions, including use of low protein diet (LPD), have been implemented as part of the primary management of CKD. However, evidence on the effectiveness of LPD in people with advanced (stages 2-4) CKD have not been appreciated by many clinicians (Kalantar-Zadeh & Fouque, 2017). Additionally, it is expected that the healthcare costs associated with CKD are expected to grow and such, measures to curb the condition are urgently needed.
Problem statement
In animal studies, the use of low protein diet (LPD) to slow the progression of renal failure is well established. However, there seems to be no consensus on whether this intervention could be effective in human population due to the modification of diet in renal disease (MDRD) (Di Iorio, Di Micco, Marzocco, De Simone, De Blasio, Sirico, Nardone, and UBI Study Group (2017). According to Iorember (2018), it is believed that in all cases of CKD, the use of LPD could help to retard the progression of the disease thereby delaying the need for renal replacement therapeutic (RRT) measures. The problem, however, is that majority of clinicians are still afraid and reluctant to prescribe LPD to individuals due to the possibilities of worsening the nutritional status of individuals. Kalantar-Zadeh and Fouque (2017) illustrated that such instances are always true in patients with advanced CKD, especially stages 2-4, and are thus showing symptoms of significantly reduced protein and calorie intake. Therefore, while there is evidence that a series of measures, can be implemented by clinicians to retard the progression of CKD, the prescription of LPD is still highly underestimated and underappreciated among primary care offices. There is need, therefore, to collate evidence from the literature on the effectiveness of LPD on how it can retard the progression of chronic renal failure. The aim is to provide clinicians with an evidence-based practice (EBP) perspective so that they can begin to appreciate the intervention in enabling patients overcome or slow the progression of their CKD.
Aim
To review the effectiveness of LPD on the progression of renal failure in humans and to recommend how to apply it in practice
Specific Objectives:
- To investigate whether LPD prevents and corrects the symptoms and complications associated with advanced CKD in humans
- To explore the effects of LPD interventions on the nutritional status of individuals with advanced chronic kidney disease
Research question
- Does the prescription of LPD to patients with advanced chronic kidney disease help to retard the progression of the condition?
- How does the administration of LPD on patients with renal failure affect their protein and calorie intake?
Significance of the review
The findings from this review are important to clinicians because it contributes to evidence-based. The findings will provide evidence on whether clinicians can safely utilise LPD in patients with advanced CKD without causing further derangement on their protein and calorie intakes. As such, the study will contribute to enhancing the safety of healthcare practices for dealing with patients with CKD.
Background
According to the NHS, a chronic kidney disease is a long-term condition where the kidneys function progressively decline, and although it can affect anyone, it is mostly associated with adult population (NHS, 2021). Watanabe (2017) defined CKD as a condition associated with reduced glomerular filtrate rate (GFR) and increased excretion of urinary albumin. Overtime, the condition could worsen and consequently lead to the total failure of the kidney function, though this is uncommon. Carrero et al. (2018) observed that the complications associated with the condition include cardiovascular mortality, acute injury to the kidneys, decline in cognitive abilities, fractures, anaemia and bone and mineral disorders. Rhee et al. (2018) noted that during the early stages, the chronic renal failure, or the CKD may asymptomatic and as such may only be diagnosed in individuals when they take urine or blood test for other reasons. As CKD advances, symptoms such as tiredness, feeling sick, shortness of breath, blood in the urine, and swollen ankles may be reported by patients. Thomas (2016) reported that the CKD is usually caused by other conditions which increases the haemostatic capacity of the kidneys. The traditional causes of CKD include hypertension, diabetes, and hyperlipidaemia. However, infections of the kidney such as nephritis and pyelonephritis, glomerulonephritis, conditions that cause obstruction to the flow of urine such as benign prostatic hypertrophy, and the regular use of medications such as non-steroidal anti-inflammatory drugs and lithium for the management of some psychiatric conditions have been implicated as contributory causes of CKD. Recently, Watanabe (2017) concluded that, diabetes mellitus is the major cause of CKD globally, with 25-35% of people with diabetes developing CKD.
CKD can be classified into five stages based on the extent of damage to the kidney. This literature review focuses on CKD stages 2-4. Table 1 describes the different stages.
| CKD stage | Explanation | Level of GFR |
| 1 | There is a normal functioning of the renal system. However, urine tests show that there could be a kidney disease | Over 90ml/min |
| 2 | There is slight reduction in the functioning of the kidney. Urine tests also show that kidney disease exists | Between 60 and 80 ml/min |
| 3A
and 3B |
A reduction in kidney functioning is observed | Between 30 and 59 ml/min |
| 4 | The kidney functioning is severely reduced | Between 15 and 29 ml/min |
| 5 | A severe kidney failure associated with ESRD | Lower than 15 ml/min. also dialysis. |
Table 1: The stages of renal failure (Source: Kidney Care UK, 2021)
According to the 2015 NICE guidelines, the progression of CKD can be defined as over 35% decrease in GFR accompanied with subsequent change in GFR category within 12 months (NICE, 2015). When estimating GFR, a clinician considers 3 GFR estimations within a period of more than 90 days. For people with reduced GFR, the estimations is taken every 14 days so that the causes of acute deterioration can be excluded. NICE (2015) guidelines further state that people who have a sustained 25% decrease in GFR for 12 months or a reduction of GFR 15ml/min/ 1.73m2 are at an increased risk of progression to End Stage Kidney Disease (ESKD). NHS (2017) observed that an estimated 6% of men and 7% of females in the UK currently suffer from stages 3-5 CRF. The Kidney Care UK recently report in 2021 reported that every year, over 3000 kidney transplants take place but an additional 5000 are still kept waiting. These statistics have shown that the renal failure problem is one of the prevalent healthcare conditions not only in UK but globally.
CKD represents public health problems to many healthcare systems across the world. Yamamoto, Nishi, Tomo, Masakane, Saito, and others (2017) illustrated that the problem is more pronounced in socioeconomically deprived countries where social inequities and lower economic statuses correlates to higher incidence of ESKD and progressive loss of renal function. According to Kidney Care UK (2021), approximately 3 million people in UK have CKD.
The NICE guidelines has offered several interventions that can be offered to people with acute renal failure. Some of these include information and education outreach, lifestyle advices, dietary interventions, LPD, pharmacotherapy, and self-management. The focus of the current paper, however, is LPD.
The role of LPD and diet interventions
According to Wu (2016), dietary measures are some of the oldest interventions for the management of CKD. The effectiveness of LPD diet in reducing the progression of CKD in animals is well documented. Although LPD are effective, many clinicians failed to control the dysfunction of renal system due to the belief that a protein intake of 0.8g/kg body weight is already a LPD (Di Iorio et al., 2017). There are many clinical trials and reviews examining what constitute an effective optimal protein intake among people with renal conditions. A study by Ko, Obi, Tortoricci, Kalantar-Zadeh (2017) reviewed the intake of protein on progression of CKD and found that protein intake varied with stages of CKD. Intake among people with advanced CKD in the United States was found to be 1.04 g/kg·Ideal Body Weight (IBW)/day or 0.81 g/kg· ABW/day. Among the healthy population, protein intake was found to be 0.8g/kg·IBW/day. Similar findings were also observed in the study by Watanabe (2017) who noted that in Japan, the intake should be +/-2 around the mean intake of 0.65 g/kg IBW. Wu (2016) also agreed with these observations and stated that currently, the agreed protein intake for healthy humans should be 0.8 g protein per kg body weight (BW) per day. The implications of these findings is that chronic intake of high or low proteins could result in renal and digestive abnormalities. In all the studies, however, there is a consensus that the intake of low proteins could result in slower progression of renal related conditions. Despite this evidence, however, many clinicians are still sceptical about the optimal protein intake prescription for people with different stages of CKD.
The effectiveness of LPD in retarding the progression of CKD have also been widely addressed in the extensive literature. A study by Bellizzi, Cupisti, Locatelli, Bolasco, Brunori, Cancarini, Caria, De Nicola, Di Iorio, Di Micco, and Fiaccadori (2016) described the pathophysiological rationale for CKD nutritional treatment of CKD among Italian population. The authors discovered that within this population, the main motivation of nutritional measures including LPD was to reduce the progression of renal failures and to ensure a better control of blood pressure. In this study, it was observed that LPD slows the growth of CKD by regulating blood pressure and increasing production of proteinuria. Similar findings have also been discovered in Brazil. In a study by Black, Anjos, JCardozo, Carmo, Dolenga, Nakao, de Carvalho Ferreira, Rosado, Eduardo, and Mafra (2018), found that LPD helps lower the progression of CKD by lowering the production of uraemic toxins that usually lead to kidney inflammations. Kalantar-Zadeh et al. (2020) agreed with these assertions and observed that the prescription of plant-dominant LPD can slow progression of CKD by altering gut microbiome which in turn helps the body to regulate the generation of uraemic toxins. Limwannata et al (2021) similarly found that dietary advice including the use of low-density lipoprotein was an effective intervention of improving serum albumin, malnutrition inflammation score, and dietary energy that in turn helped to maintain haemodialysis. Therefore, there is evidence in the extant literature that LPD can help to reduce the progress of CKD.
Gap in literature
Although there is a plethora of evidence that LPD can help to reduce the progression of CKD, the implementation of this intervention among many clinicians are still low (Bellizzi et al., 2016). Majority of the renal healthcare professionals do not have evidence of the most appropriate protein intake that should be prescribed for patients diagnosed with CKD stages 2-4 without compromising their optimal intake requirements (Bellizzi et al., 2016). Additionally, the literature shows that the intervention is not highly welcome because clinicians are not aware of its effectiveness. There is need, therefore, to review the literature, from a UK perspective, to establish the effectiveness of this intervention and to encourage clinicians to continue prescribing LPD as a therapy whether sole or adjunctive, for the management treating renal failure.
Methods
Aims and objectives of the review
This review’s main aim was to examine the extent to which the administration of LPD intervention on people with advanced CKD can help to slow the progression of the condition, thereby increasing the quality of life. As such, the study was based on two specific objectives. First, the review investigated whether LPD prevents and corrects the symptoms and complications associated with advanced chronic renal failure in humans. Second, the study explored the effects of LPD interventions on individuals’ nutritional status with progressive chronic kidney disease.
Keywords
All the keywords and phrases used were identified from the research objectives and questions. Some of the keywords and phrases included low-protein diet, chronic kidney disease, acute renal failure, chronic renal failure, CKD progression, and nutritional status of people with CKD. Additionally, a series of Boolean operators “AND” and “OR” were used to combine these keywords to generate phrases that could be searched from the identified databases. All the keywords were obtained from the PICO table 2.
Databases searched
The data were obtained from three databases, including PubMed, Medline, and Cochrane library. These databases are widely recognised in the nursing field and, as such, were trusted to provide the most accurate and reliable materials for review.
Inclusion/exclusion criteria
Table 3 below shows the inclusion and exclusion criteria used to identify the most suitable materials for this review.
| criteria | Inclusion | Exclusion | Justification | |
| Abstract | Abstract available | Abstract Unavailable | To ascertain if selected articles are relevant to research question. | |
| Year of publication | Materials published in the past five years | All materials published before 2015 | There was need to use current information in this review and materials published in the last five years were considered as more appropriate. | |
| Language | Only materials published in English were used | All materials published in languages other than English were excluded to help avoid errors and biases associated with commission and omission. The avoidance of such errors can make the findings from this review more reliable thus inferable. | ||
| Nature of the materials | Only peer reviewed materials were included in this study. | Books, articles from magazines, dissertations, thesis, and forms of grey literature were excluded | The exclusive use of peer reviewed articles was necessary because it helped to ensure that the review was based on reliable sources which were produced after undergoing through rigorous scientific methods. | |
| Population/sample | All materials with human population as the sample of studies were included | Materials which had other populations other than humans were excluded. Therefore, all studies that examined the use of LPD in rats were excluded | This criteria was necessary because it helped to address the research question about the use of LPD among human population with advanced CKD | |
| Intervention | Studies where the main intervention as LPD were included. Also, where LPD was supplemented with ketoanalogues were included | Studies which did not address LPD interventions or LPD alongside ketanalogues were excluded | Ketoanalogues supplements alongside LPD have been found to have the same and such, studies that addressed this area were found to be reliable | |
| Study design | Both quantitative and qualitative designs were included | All kinds of reviews including systematic, scoping, or traditional literature reviews were excluded |
Table 3: The inclusion and exclusion criteria
Data extraction process
First, the keywords, combined with appropriate Boolean operates, were keyed in the identified databases’ search engine. The titles were then examined, and those that did not contain any of the previously identified keywords were ignored. The remaining articles’ abstracts were then reviewed to establish their purpose, research designs and population sample, and findings. Only materials that met the inclusion criteria were moved to the next stage. Also, materials that did not have full-texts, or those that required additional login details were excluded at this stage. In the subsequent phase, the author read the articles at least two times, highlighting the keywords and creating codes relevant to answering the research question.
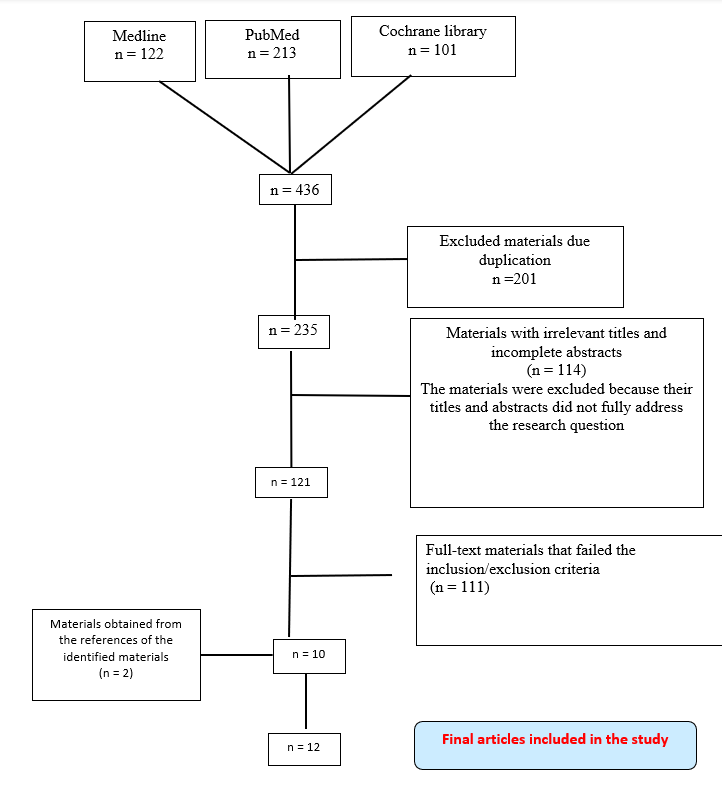
PRISMA chart
Figure 1 below illustrates the data extraction process. Based on the PRISMA process, the initial search from the three databases yielded 436 materials. However, 201 of these were considered as duplicates as were excluded from further review during the initial stage. The titles and abstracts of the remaining 235 articles were further scrutinised, a process that resulted in 114 materials being discarded for failing to meet the evaluation standards. 121 materials were then subjected to the eligibility criteria, resulting in the exclusion of a further 109 articles. The remaining 10 articles were then scrutinised, and this process helped obtain additional 2 materials from their reference lists. In total, 12 materials were found to be eligible for review. The materials were then summarised, as shown in the literature summary appendix 1.
Figure 1: PRISMA diagram
Rationale for the critical appraisal tool
The review materials were appraised, as shown in appendix 1: the literature summary table. The tool was considered vital because it guided the researcher in establishing whether the identified materials met the inclusion/exclusion criteria. The device was also essential as it helped to determine whether the findings in the specified materials succinctly helped achieve the research objectives. As such, the tool was critical in identifying the potential threats to the validity of the review. The tool also helped to show evidence about the transparency of the review, thereby allowing readers to make an evidence-based informed decision regarding all evidence presented in the review.
Data analysis technique
A thematic data analysis technique was used to analyse the information obtained from the identified materials. According to Belotto (2018), the method involves analysing qualitative data in texts or transcripts by closely examining them to identify common themes that repeatedly come up. In the current review, the technique involved six steps. In the first step, also known as the familiarisation phase, the researcher went through the collected sources to get familiar with them. The step also involved taking initial notes. The second step, known as coding, involved highlighting some sections of the identified materials to develop shorthand labels known as codes. The process also involved going through each material’s texts and then highlighting every idea that came up as important and exciting. In the third step, known as the generation of themes, the researcher looked over the created codes to establish what they had in common, a process that resulted in the creation of articles. Table 4 below shows how the codes were turned into themes. At this stage, codes that were regarded as too vague were discarded. Shorter codes were combined to generate themes. The fourth step involved reviewing the themes to represent the data in the materials reviewed accurately. After this, the themes were then defined and named in the fifth step. The main activity involved accurately formulating what each theme meant and then figuring out how it contributed to the research question. The final step involved writing up the information, as shown in the results chapter.
Findings
The findings were obtained from 14 peer-reviewed journal articles obtained after rigorously testing them through the eligibility criteria. After analysing data from these sources, two major themes emerged. The first theme was derived from the first study objective, and it was named the “effectiveness of LPD on the progression of CKD.” The second theme revolved around the second study objective and was called the “Effects of LPD on individuals’ nutritional status with CKD.” The themes areas are discussed in the following subsections.
Theme 1: The effectiveness of LPD on the progression of CKD/CRF
Based on the analysis of data obtained from the reviewed materials, there is a consensus in the literature about the effects of a low protein diet on CKD progression. In all the studies, it was found that LPD significantly retards the progression of CKD. Yen, Fan, Lee, Kuo, Tu, Chen, Lee, Hsu, Tian, and Chang (2020) examined the effects of dietary therapy on renal function progression decline using data from the Chang Gung Research Database. The findings in this study showed that even among patients with low glomerular filtrate rates, the administration of a low protein diet effectively delayed dialysis requirements. The results are significant because they confirm that LPD can effectively reduce the progress of CKD. Similar findings were also discovered in other studies reviewed. For example, Nakao, Kanazawa, and Takahashi (2018) examined the long-term effectiveness of a low-protein diet on end-stage renal failure patients. The programs were instituted among 112 patients, and observations made over four years. The findings showed that the low protein diet program was a favourable therapeutic modality for patients with ESRF. However, the intervention must be supported by trained medical staff, including nutritionists, to ensure that it is useful in the long-term. Similarly, Metzger, Yuan, Haymann, Flamant, Houillier, Thervet, Boffa, Vrtovsnik, Froissart, Bankir, and Fouque (2018) evaluated the effects of LPD on CKD’s clinical parameters. After performing a longitudinal study on 16 participants with CKD, the authors found that LPD helped improve the CKD clinical outcomes by modifying the gut microbiota and metabolic parameters. In this case, the LPD intervention was used to slow the progression of chronic kidney disease in patients.
The findings in the study by Garneata, Stancu, Dragomir, Stefan, and Mircescu (2016) also agreed with these observations. In this study, the authors performed a retrospective study of the efficacy and safety of a LPD supplemented with a ketanalogue vegetarian diet on CKD progression. The authors found that LPD interventions, augmented with a ketanalogue diet, could defer chronic kidney disease initiation among the patients. Similar findings were also discovered by Jhee, Kee, Park, Kim, Park, Han, Kang, and Yoo (2020), who, after examining the effects of protein dietary on CKD patients, found that the relative risk for renal hyperfiltration was significantly lower in patients under LPD than in the population with a high protein intake. The authors concluded that a lower protein intake among CKD patients could slow the damages to the renal hyperfiltration capabilities. Further studies by Lai et al. (2019) explored the association between high protein diets and kidney function. Using data from 9226 Korea, the authors found that a high protein intake was associated with an increased risk in renal hyperfiltration and a subsequent decline in renal function, while a low protein diet led to a reduction in the progress of renal decline. The authors concluded that patients with CKD should not be taken through a high-protein diet as this would produce deleterious effects on their renal function.
Rizzetto, Leal, Bastos, Fouque, and Mafra (2017) performed a retrospective study on 321 CKD patients to establish the effects of a LPD on their renal function. The authors demonstrated that strict adherence to a LPD was associated with improved serum creatinine, which helped slow the rate of renal failure. Similar findings were discovered in the study by Noce, Vidiri, Marrone, Moriconi, Bocedi, Capria, Rovella, Ricci, De Lorenzo, and Di Daniele (2016), who evaluated the relationship between LPD and onset of malnutrition among stages 3 and 4 CKD patients. After examining 41 patients under the LPD program, the authors discovered that the intervention helped slow down kidney disease progress. Finally, Bufarah, Costa, Losilla, Reis, Silva, Balbi, and Ponce (2018) agreed with these findings when examining the association between nutritional parameters such as protein intake with the rates of or mortality among patients with acute renal injury/CKD. After evaluating 595 patients, the authors discovered that a low protein intake alongside low albumin values was associated with higher renal injuries and failures. Among all the reviewed studies, the study Bufarah et al. (2018) was the only one that showed a negative association between low protein intake and renal functioning decline. All the reviewed studies showed that LPD could reduce the progression of renal failure among CKD patients.
Theme 2: The effects of LPD on nutritional status of individuals with CKD/CRF
Based on the data obtained from the reviewed articles, there is no consensus on how the administration of LPD affects individuals with chronic kidney disease’s nutritional status. While some studies found that the administration of LPD did not have any nutritional implications on this population, other reviews established that administering LPD could have profound health implications on patients with CKD. A study by Bellizzi, Calella, Hernández, González, Lira, Torraca, Arronte, Cirillo, Minutolo, and Cárdenas (2018) examined the impact of LPD on patients’ nutrition with CKD. The authors classified a low protein diet consisting of between 0.5g/kg/d and 0.6g/kg/d. The authors also classified a normal-high energy diet as between 30 and 36 kcal/kg/d. after comparing these values on individuals with CKD and those without, the authors found a decline in the amounts of serum urea and phosphates after six months, and the effects persisted for more than three years (Bellizzi et al., 2018). The authors concluded that within patients with CKD, the administration of LPD supplemented with ketoacids helped improve diabetes and uremia, leading to a sudden decline in body weight. The intervention did not produce any adverse effects on the patients’ muscle mass and wasting. Therefore, in this study, it was found that LPD was a safe intervention for CKD people as it did not adversely interfere with their nutritional status. Similar findings were also discovered by Garneata, Stancu, Dragomir, Stefan, and Mircescu (2016), who demonstrated that the administration of LPD alongside ketanalogue diet neither changed the nutritional parameters nor produced adverse reactions on patients with CKD. This study also showed that LPD intervention was a nutritionally safe strategy for dealing with CKD progression. Satirapoj, Vongwattana, and Supasyndh (2018) also agreed with these findings. In their study, the authors performed a retrospective cohort study to determine the progression of renal failure as well as nutritional and metabolic status among patients with stages 3 and 4 CKD under deficandin diet (VPLD). After examining data obtained from 140 participants, the authors found that there the delayed progression of CKD also contributed to preserving the nutritional status of CKD patients. This study’s findings were significant because they demonstrated that an LPD does not cause adverse interference with CKD patients’ nutritional status.
However, the findings were contrasted in the studies by Tauchi, Hanai, and Babazono (2020) and Noce et al. (2016). Tauchi, Hanai, and Babazono (2020) examined the effects of decreased protein intake on the mortality and progression of renal decline among CKD patients. After performing a single historical cohort study on Japanese patients, the authors found that the decreased protein intake increased mortality rates among CKD patients with malnutrition. This study was significant because it showed that an LPD could result in catastrophic impacts when used among CKD patients with malnutrition issues. Similar findings were discovered by Noce et al. (2016), who found that LPD programs significantly worsened the nutritional state of patients with CKD. In conclusion, the results have shown that there is mixed evidence on the effects of LPD on patients’ nutritional status with CKD.
Discussion and Conclusion
Main findings of the study
The findings have shown that LPD interventions are effective at delaying the progress of renal decline among people with CKD. The results have shown that LPD interventions are effective irrespective of the CKD stage one is at. However, the review has demonstrated that there is mixed evidence on the impacts of LPD on the nutritional status of individuals with CKD. While some studies did not find any adverse impact, other studies found that the administration of LPD interventions could interfere with individuals’ nutritional status especially if they have previously been diagnosed with malnutrition.
One of the ways through which LPD intervention retards decline in renal failure is by reducing the risk of decline of the glomerular filtrate systems. The findings have also shown that LPD helps to reduce the decline of CKD progression by modifying the microbiota and modulating the metabolic and inflammatory parameters of CKD patients (Lai et al., 2019). The findings have shown that the lower the baseline dietary protein intake (DPI), the slower an individual’s CKD condition progresses towards end-stage renal decline. However, the results have also shown that when ESRD and DPI are compared, it is impossible to obtain an optimal VPI range (Metzger et al., 2018). Based on the findings from this review, the LPD intervention is most effective when used in collaboration with an equivalent low-salt intake. The findings imply that to achieve the best results from LPD interventions, it is best when the patients are encouraged to also persistently include less-salt in their foods (Nakao, Kanazawa, & Takahashi, 2018). Also, the findings showed that LPD are most effective when combined with ketoanalogue consisting of very low proteins. The findings showed that a strict adherence to LPD retards the progress of CKD because it enhances serum creatinine as well as the rates of glomerular filtrate rates, factors associated with better renal function (Rizzetto, Leal, Bastos, Fouque, & Mafra, 2017).
The findings showed that LPD interventions worsens the nutritional status of individuals because it causes a significant reduction of serum albumin values. Also, LPD interferes with the fat-free body mass percentage that in turn leads to loss of total body water (Noce et al., 2016). The findings in the study by Noce et al (2016) also showed that the body mass cell of individuals under LPD can significantly decrease within the first six weeks, and this in turn worsens their nutritional status
Main findings in relation to previous research
The findings in this review favourably compare and contrast with previous research. The findings show that when administering the LPD on patients with CKD, it is necessary to include other stakeholders such as nutritionists to avoid committing fatal errors that can decrease the quality of healthcare outcomes. The findings are consistent with the assertions by Ko et al. (2017) who argued that an effective LPD should involve a multi-stakeholder approach where the physicians, nurses, and nutritionists all collaborate to determine the best balanced diet for any specific patient with the CKD condition. The authors argued that an LPD intervention should follow an individualised and balanced diet approach which must be consistently be evaluated by dietician to optimise its effectiveness. Ko (2017) further added that in as much as a low-protein diet could be effective, it is necessary to ensure that such diets contain adequate protein and energy to correct the energy-protein wasting. Similarly, Bellizzi et al. (2016) argued that the aim of LPD should be to implement a wider nutritional therapy rather than just focusing on decreasing the intake of proteins. Therefore, clinicians should also pay attention to issues such as a patient’s energy intake and the quality of proteins prescribed for individual patients.
The findings have shown that consistent administration of balanced LPD to patients with CKD can reduce the need for early dialysis and that this in turn could result in significant costs savings. The finding is consistent with the assertions by Watanabe (2017) who estimated that in Japan, for example, the costs of dialysis due to increased rates of CKD is estimated at 1.25 trillion Japanese yen annually. However, these would significantly reduce if clinicians begun to actively prescribe LPD for their patients.
The findings have shown that LPD intervention helps to delay the onset of dialysis by slowing the production of uremic toxins. The result is consistent with the findings by Bellizzi et al. (2016) who argued that within the Italian societies, low protein diets have been previously implemented by nephrologists as a way of alleviating uremic symptoms, slowing down the CKD progression, enhancing the start of dialysis, and delaying the commencement of dialysis. Similar assertions were also advanced by Kalantar-Zadeh and Fouque (2017) who argued that reducing the consumption of proteins helps patients to proportionately reduce the generation of urea. The authors illustrated that once proteins are broken down, an n α-amino group is then removed and this in turn helps to deactivate individual amino acids. Consequently, the remaining skeletal ketoacid can then be recycled to produce additional amino acids while the proteins are used to generate energy through the tricarboxylic cycle. This way, individuals can them stop the production of uremic toxins, and this means that the transition of dialysis to the next steps is delayed.
The results have shown that LPD interventions are most effective when supplemented with ketoanalogue supplements.
Furthermore, the findings are consistent with the assertions by Limwannata et al (2021) who demonstrated that a low protein diet in people with CKD was most effective if it was administered alongside Oral nutritional supplementation (ONS). In their study, Limwannata et al (2021) found that patients exhibited significant improvements in their intake of energy, protein, and magnesium as a result of adhering to the LPD intervention programs. Therefore, using the ONS and LPD can help to slow the progress of renal function decline by enhancing serum albumin and dietary energy among the patients.
The findings show that LPD interventions should be implemented alongside low-salt intake strategies to get the best results. The finding is consistent with the arguments by Di Iorio et al. (2017) who found that using very low proteins found in plants are most effective because they are also associated with low intake of salts and metabolic acidosis. Plant diet contain low plasma bicarbonate levels which are associated with significantly low renal deaths and mortalities. The findings, therefore, shows that diets with rich vegetables and fruits is one of the ways of implementing a LPD intervention among persons with CKD. Similar findings were also discovered by Limwannata et al (2021) who argued that the use of plant proteins instead of the animal proteins is beneficial because they can help to reduce the daily production of urinary phosphates and serum, thereby leading to low sodium and acid loads.
Policy and practical implications of the main findings
The findings in this review have significant practical and policy implications. One of the practical implications of this review is that the LPD interventions among people with CKD is effective and safe, and nurses should begin administering it to their patients. The findings from the review, therefore, contributes to evidence-based practice (EBP) as they can be used as an alternative intervention to pharmacological approaches that are always associated with adverse negative effects on patients. The review also provides evidence on how nurses can help reduce the costs of healthcare to people with CKD as LPD interventions are much cheaper than the drugs/medicines. For example, since it has been obtained that LPDs retards the need for early dialysis, this subsequently leads to significant costs savings. The review, therefore, can be used to dispel worries among the nurses about the effects of LPD interventions on the overall health of patients.
One of the major policy implications of this review is that there is need for healthcare systems such as the NHS to begin sensitising their healthcare personnel about the importance and efficacy of LPD interventions among patients with different stages of CKD. Such sensitisation and awareness programs can be included in the curriculum and be examinable. Most importantly, the NHS policy makers should device the most appropriate amounts of LPD interventions that can be administered to achieve the optimum outcomes for each stage of CKD.
Strengths and limitations of the review
The major strength of this review is that it was based on the most current peer reviewed articles which provided results from empirical studies. According to Durkin et al. (2018), peer reviewed articles are based on expert knowledge and also prevents the publication of falsified work. As such, the findings from this review are more valid and reliable, and can be used as source of evidence-based practice.
As with any other desk-based research, the current review was limited to use already published data. Usually such types of data are characterised as having high possibilities of being incomplete and unreliable. Therefore, this study can be improved by collecting and analysing empirical data. The second limitation in this review is that it did not review he LPD needs of individuals in different stages of CKD. There is evidence that the different stages of CKD are associated with different symptoms and should be addressed with specific medications which may vary from each other in terms of their efficacy, intensity, and frequency. The current study did not show the right amount of LPD that must be prescribed from each patient. Future empirical studies should, therefore, the proteins needs of patients at every stage of CKD to optimise their effectiveness and enhance healthcare outcomes for patients.
Recommendations and perceptive for practice and future research work
It is recommended that for practice, clinical personnel including nurses should consider administering LPD interventions among their patients with various stages of CKD. However, care should be taken when dealing with patients with other underlying conditions such as malnutrition to avoid committing significant adverse impacts on their health.
It is recommended that future researchers should focus their attention on examining the impacts of LPD interventions on the different stages of CKD patients who have previously been diagnosed with different conditions such as malnutrition. In the present review, only one study addressed this area, and the results show that in such population, LPD interventions could produce adverse effects. Therefore, there is need for more evidence in this area, and future studies can help bridge the gap.
Appendices
Appendix 1: Literature review summary table
| Authors(Year) | Study design | Sampling strategy | Data collection method and analysis | Key findings | |
| Bellizzi et al. (2018) | observational prospective study | Purposive sampling consisting of 197 stages 3-5 CKD patients | Observation | The use of LPD, accomplished with ketoacids, helped to enhance uremia and diabetes among patients with CKD. There was a sudden decline in body weight. There was no adverse effects on the muscle mass and fitness of patients. | |
| Bufarah et al. (2018) | prospective observational study | Purposive sampling consisting of 64 ICU patients aged between 54 and 75 years old. | Observation | Low calorie and protein intake was associated with negative nitrogen balance among patients with acute renal injury/failure | |
| Garneata et al. (2016) | prospective, randomized, controlled trial | N/A | Observation | There were no adverse reactions when people with CKD were given LPD. Very-low protein diet was found to be safe and could retard the progression of CKD | |
| Jhee et al. (2020) | prospective study | Purposive sampling consisting of 9226 subjects | Observation of data from 9226 patients in Korean Genome and Epidemiology | Low protein diet was associated with low risks of renal hyperfiltration and decline | |
| Lai et al. (2019) | longitudinal, prospective, controlled, and interventional | Purposive sampling consisting of 16 patients with CKD and under LPD | Experimentation and observation | LPD interventions can produce useful outcomes for CKD patients because it helps to modulate the microbiome of individuals. | |
| Metzger et al. (2018) | prospective cohort | Snowball that yielded 1594 CKD patients | Using the Maroni formula | The lower the dietary protein intake, the slower the progress of ESRD. However, there was no threshold for protein intake and ESRD, thus, no optimal low protein intake recommended | |
| Nakao et al. (2018) | Longitudinal design | Snowball consisting of 112 patients with CKD and creatine clearance of under 5.0 mL/min. | Observation | Low-protein and low-salt dietary interventions provided a favourable therapeutic for patients with ESRF. The treatment, however, could not be regarded as a general strategy for patients with ESRF | |
| Noce et al. (2016) | Longitudinal design | Random sampling technique consisting of 41 Italian CKD patients with a mean age of 73 years. | Observation | LPD helped to slow down the progression of CKD. However, the intervention worsened the patients’ nutritional state. | |
| Rizzetto, Leal, Bastos, Fouque, & Mafra (2017) | Retrospective study | Purposive sampling of 321 non-dialysis CKD patients | Observation | Adherence to LPD helped to enhance serum creatinine which delays the progress of renal failure | |
| Satirapoj et al. (2018) | Retrospective cohort study | Purposive sampling consisting of 140 patients with stage 3 and 4 CKD | Statistical measurements | Very LPD was associated with a delayed progression of renal failure while also preserving the nutritional status of patients with CKD. | |
| Tauchi et al. (2020) | single-centred historical cohort study | Purposive sampling of 449 Japanese patients with diabetes and CKD | Lower dietary protein intake was associated with reduced incidences of renal replacement therapy initiation, an indication of beneficial effects of LPD on kidneys. However, it led to higher mortality rates among patients with malnutrition. | ||
| Yen et al. (2020) | retrospective cohort study | Random sampling consisting of 3282 participants | Observation of data from Chang Gung Research Database (CGRD | LPD interventions effectively delayed progression of CKD even among patients with low estimated glomerular filtration filtrates |
Appendix 2: The coding process
| Codes | Themes |
| CKD progression, defer, LPD, glomerular filtrate decline, | The effectiveness of LPD on the progression of CKD/CRF |
| Nutritional status, malnutrition, LPD, | The effects of LPD on nutritional status of individuals with CKD/CRF |